High Energy Electron Diffraction
advertisement

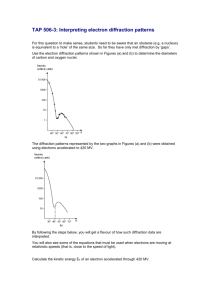
High Energy Electron Diffraction Research and explain how electron diffraction can be used to determine the radius of the nucleus (6 Marks) Mark Scheme Any 6 from:1. A beam of high energy electrons is directed at a thin sheet of an element 2. and accelerated through a potential difference of about 108 volts (MeV/high energy) 3. A detector measures the number of electrons diffracted at a number of different angles. 4. Scattering effects occur due the charge of the nuclei and electron and this causes the count rate of the beam of electrons to decrease as angle increases. 5. The electrons are also diffracted by the nuclei in the sheet which causes minima and maxima to observed in the final pattern 6. as long the de Broglie wavelength of the electrons is of the same order as the size of the nucleus, which is about 10-15 m. 7. The diameter of the nucleus can be calculated using the angle to the first minimum, θmin 8. and the wavelength of the incoming beam λ using the diffraction equation R sin θmin = 0.61λ.



![The electronic configuration of phosphorus is [Ne] 3s2 3p3](http://s3.studylib.net/store/data/008974852_1-8381577ce936fbfa611892c1a5f109cd-300x300.png)