Suggested problems Chapter 2 2.31 What is the name of the
advertisement
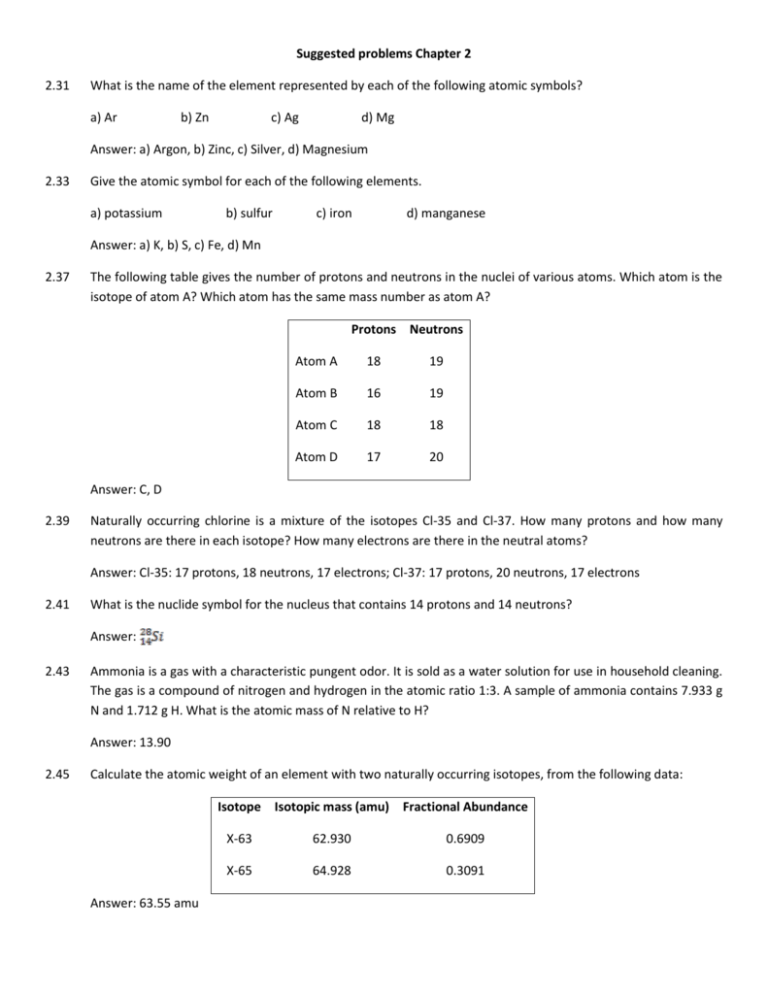
Suggested problems Chapter 2 2.31 What is the name of the element represented by each of the following atomic symbols? a) Ar b) Zn c) Ag d) Mg Answer: a) Argon, b) Zinc, c) Silver, d) Magnesium 2.33 Give the atomic symbol for each of the following elements. a) potassium b) sulfur c) iron d) manganese Answer: a) K, b) S, c) Fe, d) Mn 2.37 The following table gives the number of protons and neutrons in the nuclei of various atoms. Which atom is the isotope of atom A? Which atom has the same mass number as atom A? Protons Neutrons Atom A 18 19 Atom B 16 19 Atom C 18 18 Atom D 17 20 Answer: C, D 2.39 Naturally occurring chlorine is a mixture of the isotopes Cl-35 and Cl-37. How many protons and how many neutrons are there in each isotope? How many electrons are there in the neutral atoms? Answer: Cl-35: 17 protons, 18 neutrons, 17 electrons; Cl-37: 17 protons, 20 neutrons, 17 electrons 2.41 What is the nuclide symbol for the nucleus that contains 14 protons and 14 neutrons? Answer: 2.43 Ammonia is a gas with a characteristic pungent odor. It is sold as a water solution for use in household cleaning. The gas is a compound of nitrogen and hydrogen in the atomic ratio 1:3. A sample of ammonia contains 7.933 g N and 1.712 g H. What is the atomic mass of N relative to H? Answer: 13.90 2.45 Calculate the atomic weight of an element with two naturally occurring isotopes, from the following data: Isotope Isotopic mass (amu) Fractional Abundance Answer: 63.55 amu X-63 62.930 0.6909 X-65 64.928 0.3091 2.47 An element has three naturally occurring isotopes with the following masses and abundances: Isotopic mass (amu) Fractional Abundance 38.964 0.9326 39.964 1.000 x 10-4 40.962 0.0673 Calculate the atomic weight if this element. What is the identity of the element? Answer: 39.10 amu, potasium 2.49 While traveling to a distant universe, you discover the hypothetical element X. You obtain a representative sample of the element and discover that it is made up of two isotopes, X-23 and X-25. To help your science team calculate the atomic mass of the substance, you send the following drawing of your sample with your report. = X-23 = X-25 In your report you also inform the science team that the gold atoms are X-23, which have an isotopic mass of 23.02 amu, and the green atoms are X-25, which have an isotopic mass of 25.147 amu. What is the atomic mass of the element X? Answer: 24.615 amu 2.51 Identify the group and period for each of the following. Refer to the periodic table (Fig 2.15 or inside front cover). Label each as a metal, nonmetal, or metalloid. a) C b) Po c) Cr d) Mg e) B Answer: a) Group IVA, period 2, nonmetal; b) Group VIA, period 6, metal; c) Group VIB, period 4, metal; d) Group IIA, period 3, metal; e) Group IIIA, period 2, nonmetal 2.59 A 1.50 g simple of nitrous oxide (an anesthetic, sometimes called laughing gas) contains 2.05 x 1022 N2O molecules. How many nitrogen atoms are in this sample? How many nitrogen atoms are in 1.00 g of nitrous oxide? Answer: 4.10 x 1022 N atoms, 2.73 x 1022 N atoms 2.61 A sample of ammonia, NH3, contains 3.3 x 1021 hydrogen atoms. How many NH3 molecules are in this sample? Answer: 1.1 x 1021 NH3 molecules 2.63 Give the molecular formula for each of the following structural formulas. H H a) H H H N N H hydrazine H H H C C C H O H b) O H hydrogen peroxide H Cl P Cl H isopropyl alcohol c) O d) Cl phosphorus trichloride Answer: a) N2H4, b) H2O2, c) C3H8O, d) PCl3 2.65 Write the molecular formula for each of the following compounds represented by the molecular models. a) b) c) Answer: a) PCl5, b) NO2, c) C3H6O2 2.67 Iron(II) nitrate has the formula Fe(NO3)2. What is the ratio of iron atoms to oxygen atoms in this compound? Answer: 1 Fe to 6 O 2.87 Iron(II) sulfate heptahydrate is a blue-green, crystalline compound used to prepare other iron compounds. What is the formula of iron(II) sulfate heptahydrate? Answer: FeSO4 · 7H2O 2.89 For the balanced chemical equation Pb(NO3)2 + K2CO3 PbCO3 + 2KNO3, how many oxygen atoms are there on the left? Answer: 9 2.101 Compounds of europium, Eu, are used to make color television screens. The europium nucleus has a charge of +63. How many electrons are there in the neutral atom? In the Eu+3 ion? Answer: 63, 60 2.105 Obtain the fractional abundance for the two naturally occurring isotopes of copper. The masses of the isotopes are , 62.9298 amu; , 64.9278 amu. The atomic weight is 63.546 amu. Answer: Cu-63:0.6916, Cu-65:0.3084