Chapter 22 Living in a nuclear age
advertisement

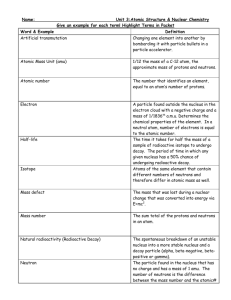
CHAPTER 22 LIVING IN A NUCLEAR AGE Name: QUESTIONS 22.1 Structure of the atom 1. Use the periodic table to determine the number of (i) protons and (ii) neutrons in the nucleus of the most abundant form of the following elements. (a) Calcium (b) Aluminium (c) Iodine (d) Sodium (e) Cobalt 22.2 Binding energy 2. Calculate the binding energy of 3. The isotope 4. 12 N 7 12 C, 6 which has a nuclear mass of 11.996706 amu. has a nuclear mass of 12.014770 amu. Calculate its: (a) mass defect (b) binding energy (c) binding energy per nucleon. Element X has an atomic number of 29, a mass number of 64 and a nuclear mass of 63.913843 amu. (a) What is the identity of element X? (b) Calculate its binding energy. © John Wiley & Sons Australia, Ltd 1 QUEENSLAND PHYSICS 22.3 Radioactive decay 238 U 92 5. The half-life of 6. Lead-212 has a half life of 10.6 hours. How much of an initial 40 g sample of this isotope will remain after: 7. (a) 21 hours? (b) 5 hours? (c) 2 days? is 4.5 billion years. What is its decay constant? When a carbon-14 test is done on human remains found in a peat bog, it is found that approximately 60% of the carbon-14 present in the body at the time of death has undergone nuclear decay. If the half-life of carbon-14 is 5730 years, how long has the person been dead? 22.5 Nuclear transmutation 8. The isotope 45 Ca 20 decays to form a new isotope, and releasing a beta particle and a neutrino. What is the new isotope? 9. What particle is released when phosphorus-30 decays to form silicon-30? 10. What new isotope is formed when a nucleus of the isotope 14 N 7 absorbs an alpha particle? Assume no other particles are emitted. Review questions Understanding 1. List alpha, beta and gamma rays in order of their penetrating power. Why is the order of their penetrating power the opposite to that of their ionising power? 2. Even though it was discovered before the nucleus was identified, radioactive decay was not thought to be a chemical reaction. What properties of radioactive decay exclude it © John Wiley & Sons Australia, Ltd 2 QUEENSLAND PHYSICS from being a chemical reaction? 3. If nuclei do not contain electrons, how can beta decay be accounted for? 4. What property of neutrons makes them particularly useful for producing nuclear reactions? 5. Which has a greater mass, a uranium nucleus before fission or the products after fission? Explain. 6. (i) What is the isotope formed when uranium-238 captures a neutron? (ii) The nucleus of this isotope is unstable because it has an excess of neutrons. What happens to it to allow it to restore stability? (iii) What is the isotope eventually formed? 7. List the things that can happen to a neutron produced in a fission of uranium-235. 8. What are some reasons why a chain reaction does not occur in a natural deposit of uranium? 9. Why was there such a large mass of graphite (about 400 tonnes) in the first atomic reactor? 10. How is the chain reaction in a nuclear reactor maintained at a steady level? Application 11. State the numbers of protons and neutrons in each of the following nuclei. (a) Aluminium-27 (b) Lead-208 (c) Radon-220 (d) Polonium-218 (e) Uranium-238 12. What changes in atomic number and mass number result from the emission of: © John Wiley & Sons Australia, Ltd 3 QUEENSLAND PHYSICS (a) an alpha particle? (b) a beta particle? (c) a gamma ray? 13. Write nuclear reactions for the following decays. (a) Polonium-214 emits an alpha particle. (b) Thallium-210 emits a beta particle. 14. Complete the following nuclear equations: (a) 27 Al 13 (b) 10 B 5 (c) 27 Al 13 (d) ? ? ? (e) 9 Be 4 (f) 22 Al 11 2 H ?? 1 n 1 ? 0 4 He ? ? 1 H 2 ? 1 4 He ? ? 1 H 2 ? 1 4 He 35 Cl 1 H 2 17 1 1 H ? ? 4 He 1 ? 2 4 He ? ? 1 H 2 ? 1 15. If it was possible for a nucleus of carbon-12 to absorb a neutron into its nucleus and form carbon-13, calculate the amount of energy that would be released. The atomic masses are: carbon-12: 12.000 000 u carbon-13: 13.003 354 u. 16. When a proton is fired at a lithium nucleus, a nuclear reaction in which two alpha particles are produced may occur. 7 Li 3 1 H 4 He 4 He 1 2 2 Given that the atomic mass of 7 Li is 7.016 003 u, calculate the total kinetic energy of the 3 © John Wiley & Sons Australia, Ltd 4 QUEENSLAND PHYSICS two alpha particles after the reaction. (Ignore any initial kinetic energy of the lithium atom and the proton.) Note: Even though we are dealing with a proton and two alpha particles in this reaction, we still use the atomic masses of hydrogen and helium. By doing so we ensure that we have accounted for the mass of the same number of electrons on each side of the equation. 17. The first artificial nuclear transmutation that Rutherford performed in 1919 was the reaction between an alpha particle and a nitrogen nucleus. The alpha particles used in the experiment were emitted from bismuth-214. 14 N 7 4 He 17 O 1 H 2 8 1 (a) Use the masses given on the following page to determine the change in energy (in MeV) that occurred in the reaction. (b) You should have noticed that energy was absorbed in the reaction. What was the source of this energy? The atomic masses are: N 14.003 074 u 14 7 17 8 4 2 O 16.999 131 u He 4.002 603 u 1 1 H 1.007 825 u. 18. Calculate the amount of energy released in the following nuclear reaction. 14 N 7 2 H 15 N 1 H 1 7 1 The atomic masses are: 14 7 2 1 15 7 N 14.003 074 u H 2.014 102 u N 15.000 108 u 1 1 H 1.007 825 u. 19. The process of nuclear fusion occurring in the Sun involves a number of steps but can be summarised in the equation 4 1 H 4 He 2 0 e 2v 1 2 1 How much energy is released in this process? © John Wiley & Sons Australia, Ltd 5 QUEENSLAND PHYSICS Challenge 20. Rutherford estimated that there was sufficient energy in 1 gram of radium to raise 500 tonnes a mile high. Presumably, 1 gram of uranium would contain similar energy, perhaps enough to raise 500 tonnes 1.6 km high. The energy output from the first uranium bomb was equivalent to about 20 kilotonnes of TNT. The mass of uranium in that bomb was about 40 kg. (The energy output of 1 kilotonne of TNT is equal to 4.2 1012 J.) How accurate was Rutherford’s 1903 estimate? Notes: © John Wiley & Sons Australia, Ltd 6