Mech 473 Homework Assignment #1
advertisement

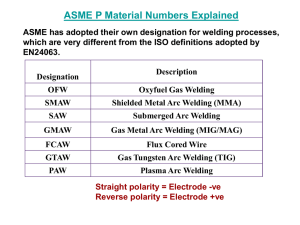
Mech 473 Homework Assignment # 3 1. You graduate from UVic and are hired by a NASA contractor to place a three-foot diameter microsatellite into orbit. The satellite will contain delicate electronic equipment that will send and receive radio signals from the earth. Design the shell within which the electronic equipment will be contained. What properties will be required and what kind of materials might be considered. (1) 2. The hull of the space shuttle consists of ceramic tiles bonded to an aluminum skin. Discuss the design requirements of the shuttle hull that led to the use of this combination of materials. What problems in producing the hull might be designers and manufacturers have faced. (1) 3. Aluminum has a density of 2,7 g/cm3. Suppose you would like to produce a composite material based on aluminum having a density of 1.5 g/cm3. Design a material that would have this density. Would introducing beads of polyethylene or hollow glass spheres, which have a density of 0.95, into the aluminum be a likely possibility. Explain. (1) 4. Explain why aluminum alloys containing more than about 17% Mg are not used. Assume that the phase is an intermetallic compound and a eutectic structure is produced. (1) When more than 17% Mg is added to Al, a eutectic microconstituent consisting of the phase and a brittle phase is produced during solidification as seen in its phase diagram. This eutectic contains 35.0 17.4 % 97.2% 35.5 17.4 Most of the eutectic is thus the brittle intermetallic compound and it will likely embrittle the eutectic. The brittle eutectic, which is the continuous microconstituent, will make the entire alloy brittle. 5. Based on their phase diagrams, which of the following three alloys would be most suited for thixocasting? Explain your answer using their respective phase diagrams. (2) a) Al—12%Si b) Al-1%Cu c) Al-10%Mg Alloys best suited for thixocasting are those with a large freezing range. Of the alloys listed, Al-10%Mg has a freezing range of 110 oC, which is the largest of the three and is thus the most suitable. The Al-12%Si is a eutectic (approximately 0 oC freezing range) and Al-1%Cu has a freezing range of only 10 oC. 6. Suppose a 24-in long round bar is to support a load of 400 lbs without any permanent deformation. Calculate the minimum diameter of the bar if it is made of 6061-T6 aluminum alloy. Also calculate the weight of the bar and the approximate cost. Pricing is typically based on the LME as per this link. http://www.lme.co.uk/aluminiumalloy.asp (1) Area Force Yield Strength A 400/40,000 0.01 in 2 diameter 4A/π 0.113 inches Weight 24 in 0.01 in 2 0.097 lb/in 3 Weight 0.0233 lb cost $2.76 (USD)/lb 0.0233 lb $0.06 The raw cost for the 6063 alloy (reference material) can be found at the above web site. There is then an additional cost for 6061 (approx 10 cents) and an additional cost to convert to billet (which is the raw mat'l at approx 10 cents per lb), on top of that you have the cost to convert billet to extrusion. As per the website, the current price for a cash buyer is 2340mt. (2340/2200 = 1.06/lb + .10 for 6061 + .10 for conversion to billet = 1.26 US per pound for 6061 billet. The conversion cost from billet to extrusion is typically between 1.35 & 1.65 US per pound depending on the complexity. So for 6061 aluminum extrusion, the pricing would be 1.26n + 1.50 (average conversion) = 2.76 US per pound. Above is the response from Joe Jackman of ALMAG. 7. Compare the percentage increase in the yield strength of commercially pure annealed aluminum and its 1100-H18 alloy and explain the difference. (1) 1100 H18 22,000 100% 440% 1100 O 5,000 Aluminum and its alloys have high strain hardening coefficients due to its FCC structure and thus can be cold worked a large amount due to their good ductility. 8. Calculate the density of a cemented carbide, or cermet, based on a titanium matrix if the composite contains 50 wt% WC, 22 wt% TaC and 14 wt% TiC. (See Asklande & Phule, example problem 16-2 4th edition or 17-2 5th edition for densities). (2) 9. (2) 10. A polyester matrix with a tensile strength of 13,000 psi is reinforced with Al2O3 fibers having a tensile strength of 300,000 psi. What vol% fibers must be added to insure that the fibers carry 75% of the applied load? (1) 11. (2) 12. The company that you are working for welds aluminum parts together using the GMAW (MIG) process. Currently, they are using CO2 gas to protect the aluminum during the welding process. However, the CO2 gas is too cold and leaves a rough finish on the welds. What gas would you choose to improve the finish on the welds? Why would you choose this particular gas? (1) Answer: Choose argon gas because it does not cool the weld as quickly. (This allows the weld puddle to smooth out more before freezing.) 13. Stainless steels can suffer from “weld decay”. What is it and what two methods can be used to eliminate it? (2) Weld decay occurs in the stainless steels and is the precipitation of (CrFe)4C, which contains 70 % Cr, at grain boundaries causes the concentration of Cr in the adjacent grains to fall below 12%, which degrades the corrosion resistance properties of the stainless steels. The optimum temperature for precipitation of (CrFe)4C is around 650 oC, which is attained in the heat affected zone adjacent to a fusion weld. 14. Submerged-arc welding is used for the high-output welding of large steel panels (such as ship decks) in the flat position. Describe the Submerged-Arc Welding process. (1) Submerged-arc welding is both fast and automatic. Heat is supplied by an arc between a bare metal electrode and the work piece while the shielding is provided by a blanket of granular flux. The electrode and the flux are both fed continuously as the arc is traversed along a pre-machined vee interface. The flux melts in the vicinity of the arc and then solidifies to farm a protective cover over the cooling weld. The flux is an insulator in the solid state but become highly conductive on melting and thus maintains the submerged arc. High quality welds can be produced at high rates of weld metal deposition. The high deposition rates are achieved with narrow electrodes passing high currents, which results in a relatively coarse columnar structure and an enlarged heat affected zone causing poorer low temperature ductility.