CHAPTER 2 INTRODUCTION TO THE ATMOSPHERE
advertisement

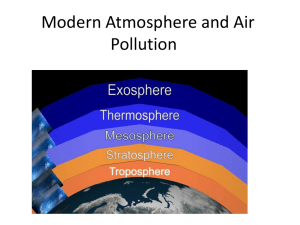
CHAPTER 2 INTRODUCTION TO THE ATMOSPHERE The atmosphere represents a place where a balance is generally achieved between the downward-directed gravitational force and the upward-directed force of buoyancy o This balance is termed hydrostatic equilibrium Atmosphere is a subset of the air because the atmosphere is solely composed of gases Air contains the gases and aerosols The total mass of the atmosphere is 5 x 1018 kg (5 x 1015 metric tons) The mass of the atmosphere is in constant motion and has considerable impact on the surface environment. Origin of the Earth and Atmosphere The atmosphere originated early in the history of the planet as volcanic materials cooled and outgassed Outgassing consisted primarily of diatomic nitrogen (N2) and carbon dioxide (CO2), with lesser amounts of water vapor, methane (CH4), and sulfur The water vapor condensed into liquid water in the cool atmosphere, forming clouds and precipitation Atmospheric Composition Today, the dry atmosphere consists primarily of N2 and diatomic oxygen (O2) Nitrogen is a highly stable gas that composes 78 percent of the present-day atmospheric volume Oxygen composes approximately 21 percent 0.93 percent of the remaining one percent is composed mostly of argon CO2 is the fourth-most abundant gas in the dry atmosphere, with 0.037 percent of the dry atmosphere The early evolution of aquatic green plants led to a significant extraction of CO 2 in the atmosphere, and photosynthesis released O2 to the atmosphere as a by-product o Ozone formed from the atmospheric O2, and from that point forward, terrestrial life was possible The Carbon Cycle Carbon follows a cycle in which it may be stored for certain periods of time within a number of reservoirs such as rock layers, the ocean, biomass, and the atmosphere o The largest carbon reservoir, comprising the vast majority of carbon in the earthocean-atmosphere system, is sedimentary rock o The second-largest reservoir is the oceans, which act as a sink for atmospheric CO2 o The biosphere (including soil) is a reservoir that acts as a temporary sink for storing the majority of CO2 from the atmosphere o The atmosphere represents the smallest carbon sink, and the carbon exchange rate to this reservoir is rapid – only about 100 years The Keeling Curve verifies the rapid increase since 1957,and also reveals the seasonal cycle of CO2 CO2 absorbs energy radiated from the earth and re-emits energy back downward toward the earth, thereby keeping the surface warmer than it would be if the CO 2 were not present o This phenomenon is known as the greenhouse effect The rapid increase in the quantity of atmospheric CO2 is the most likely culprit for the observed increases in temperature of the earth’s surface in the last several decades Constant and Variable Gases Constant gases have relatively long residence times in the atmosphere and that occur in uniform proportions across the globe and upward through the bulk of the atmosphere These gases include N2, O2, argon, neon, helium, krypton, and xenon Variable gases are those that change in quantity from place to place and/or over time o They generally have shorter residence times than constant gases o The most notable variable gas is water vapor o The amount of water vapor in the atmosphere varies dramatically across space because water and energy must be available at the surface for evaporation to occur o Other important variable gases are CO2, CH4, nitrous oxide (N2O), carbon monoxide (CO), tropospheric ozone (O3), and chlorofluorocarbons (CFCs) The Faint Young Sun Paradox There is an apparent contradiction between what is known about weak amount of energy released by our young sun, and evidence of the earth’s temperature through geologic time Methane may have kept earth temperatures within a very narrow range of variability, despite a weaker solar output Atmospheric Structure The troposphere is the lowest layer of the atmosphere o The first 8-20 km (5-12 miles) above earth’s surface o It’s the layer in which nearly all weather and climate processes of importance occur o Temperatures in the troposphere usually decrease with height The stratosphere is the second layer o It’s characterized by increases in temperature with height because of ozone absorption o The so-called “ozone layer” is in the stratosphere The mesosphere is above the stratosphere In the heterosphere, above the mesosphere, gases of the atmosphere stratify into layers according to their atomic weights The top of the troposphere is called the tropopause, while the stratopause separates the stratosphere from the mesosphere, and the mesopause separates the mesosphere from the thermosphere