Atomic Structure
advertisement

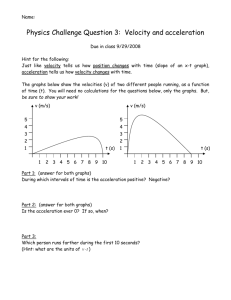
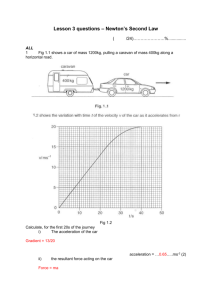
Accelerated Physical Science: Final Exam Review Topics IPS Labs 6.2 – decomposition of water (electrolysis) 6.3 – synthesis of water 6.4 – synthesis of zinc chloride Atomic Structure (study guide, quiz, test – with moles and bonding) protons, neutrons, electrons: relative mass, location, charge Definition of atomic number definition of isotopes, finding # of protons and neutrons and electrons isotope notation and mass number (mass number = #protons + #neutrons) written like this: 13C (this isotope of C has a mass number of 13 and therefore, 6 protons, 7 neutrons and 6 electrons periodic table: s and p blocks, alkali metals, halogens, noble gases, groups (vertical) vs. periods (horizontal) groups are similar because they have the same number of valence electrons flame test lab, quantization of energy, excited state vs. ground state, ROYGBIV—energy, frequency, and wavelength of each type of light wavelength and energy (short waves = high energy) wavelength and frequency—(short wavelength = high frequency) Moles (study guide, quiz, test – with atomic structure and bonding) molar mass and molecular mass (amu) Avogadro’s number (6.022 X 1023) convert g to moles, moles to g; molecules to moles, moles to molecules; g to molecules, molecules to g stoichiometry: mole ratio, limiting reactants, Balancing Equations Bonding (study guide, quiz, test – with atomic structure and moles) Chemical bond, ionic bond, covalent bond electronegativity, polar and nonpolar covalent bonds valence electrons Lewis structures, octet rule Most common ion – predicting charge from periodic table Electron configuration Hydrogen “bonding” “World of Chemistry” videos online at www.learner.org The Universe (Space take-home test, Expansion of Universe lab, Updating the Big Bang activity) Big Bang theory Doppler effect (Red and Blue Shift) Life of stars Formation of the solar system Fundamental Forces (points for discussion worksheet) Gravity (know equation), Electricity (know equation), Weak Nuclear Force, Strong Nuclear Force Know where each operates, relative strengths Inverse square law Changes in gravitational/electromagnetic forces when change mass/charge/ distance Attractive and repulsive forces Cavendish’s experiment to determine ‘G’ “Mechanical Universe” videos online at www.learner.org Physics Ch 2 Linear Motion (quiz, test with chapter 3, Lab P01 – Position and Velocity) Speed, velocity, acceleration, average speed vs. instantaneous speed scalar vs. vector quantities the three general equations: d = vt + = ½ at2 vf = vi + at vf2 = vi2 + 2ad d = vt horizontal motion, constant velocity v =at (or v = gt) finds peak time in flight d = ½ at2 (vertical distance in free fall) find velocity of an object dropped from a height, the time it takes to fall, and distance it travels in that time graphs of speed vs. time; distance vs. time; acceleration vs. time Ch 3 Projectile Motion (quiz, test with chapter 2) adding vectors together to get a resultant vector using a2 + b2 = c2, resolving vectors into two horizontal and vertical components 3/4/5 (37,53,90) and 1/1/√2 (45,45,90) triangles finding the horizontal and vertical velocities of an object shot at an angle finding the h and v velocities as an object drops to the ground a dropped bullet and a horizontally shot bullet spend the same time in the air, determined by v = at (v = gt) find how far an object can travel horizontally when fired from horizontally or at an angle find how a far an object can travel vertically when fired straight up or at an angle Ch 4 Newton’s First Law-Inertia (quiz, test with chapters 5 and 6) definition of Newton’s first law and explanation At earth’s surface, g = 9.8 m/s2 Weight = mass x acceleration due to gravity: w = mg units in kg m and s difference between mass and weight net force – vector sum of all forces acting on an object equilibrium: net force = 0 Ch 5 Newton’s Second Law—Force and Acceleration (quiz, test with chapters 4 and 6, Lab P08 – Constant Force) definition of Newton’s second law and explanation F = ma, a = Fnet/m Force directly related to acceleration, acceleration inversely related to mass no acceleration means no net force and vice versa (it does not mean no force at all, just that the forces balance; also, it does not mean no velocity, just no CHANGE in velocity) friction, pressure (force/area) terminal velocity-achieved when air resistance is equal to downward weight, velocity decreases, then remains constant when upward force = downward force (net force = zero) Ch 6 Newton’s Third Law—Action and Reaction (quiz, test with chapters 4 and 5) definition of Newton’s third law and explanation action reaction pairs— for example apple-orange and horse-cart problems Chapter 8-Energy Potential energy: PE = mgh Kinetic energy: KE = ½ mv2 law of conservation of energy says that loss in PE = gain in KE work = force x distance, W=Fd (units of joules-kg m /s2 ) work can change the KE of an object, W = KE (work-energy theorem) and can change the potential energy, W = PE energy is the “something” required to do work thus units of J are used for work and energy power is the rate of doing work, Power = work/time (units are watts or J/s)