Experimental Procedure (this uses ½ of the
advertisement
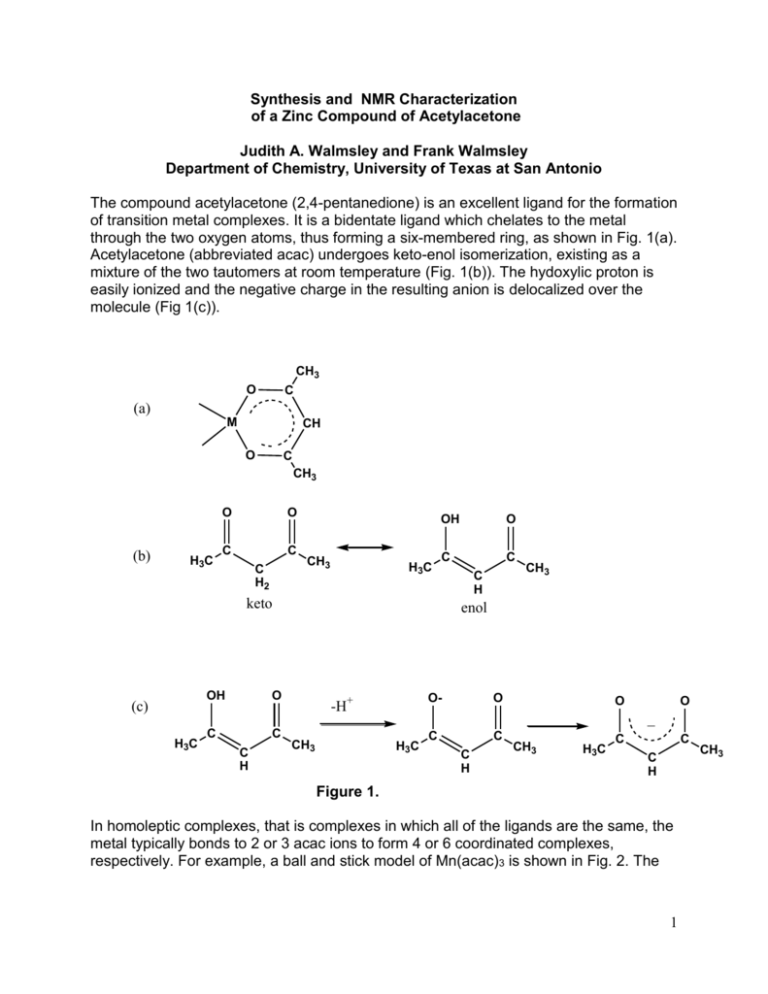
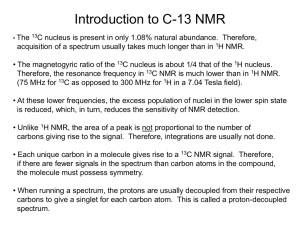
Synthesis and NMR Characterization of a Zinc Compound of Acetylacetone Judith A. Walmsley and Frank Walmsley Department of Chemistry, University of Texas at San Antonio The compound acetylacetone (2,4-pentanedione) is an excellent ligand for the formation of transition metal complexes. It is a bidentate ligand which chelates to the metal through the two oxygen atoms, thus forming a six-membered ring, as shown in Fig. 1(a). Acetylacetone (abbreviated acac) undergoes keto-enol isomerization, existing as a mixture of the two tautomers at room temperature (Fig. 1(b)). The hydoxylic proton is easily ionized and the negative charge in the resulting anion is delocalized over the molecule (Fig 1(c)). CH3 O C (a) M CH O C CH3 (b) H3C O O C C C H2 CH3 H3C OH O C C keto (c) H3C OH O C C C H C H CH3 enol -H+ CH3 O- O C C O O _ H3C C H CH3 H3C C C C H Figure 1. In homoleptic complexes, that is complexes in which all of the ligands are the same, the metal typically bonds to 2 or 3 acac ions to form 4 or 6 coordinated complexes, respectively. For example, a ball and stick model of Mn(acac)3 is shown in Fig. 2. The 1 CH3 geometry about the Mn(III) is approximately octahedral with some Jahn-Teller distortion. This complex and all homoleptic acac complexes that are octahedral are chiral. Figure 2. Mn(acac)3 octahedral complex.1 In this experiment you will synthesize and isolate a compound of Zn(II) and acac. Although the procedure is designed to yield the monohydrate, Zn(acac)2∙H2O, the anhydrous2 or dihydrate3 may be obtained. Using a variety of instrumental methods you will analyze your product to determine which compound you have made. The zinc acetylacetonate monohydrate is 5-coordinate (Fig. 3) and could have square pyramidal or trigonal bipyramidal geometry. Xray analysis has shown the structure to be square pyramidal with some distortion toward a trigonal bipyramidal structure. The water molecule occupies the axial position.4 The anhydrous acetylacetone complex of Zn(II) is a trimer containing both 5- and 6-coordinate zinc.2 Figure 3. Possible structures of zinc acetylacetone hydrates. When H2O is present in a compound as a hydrate, or a material is just wet, it will appear in the IR spectrum. If the compound contains water, a very broad band will appear in the 3200-3300 cm-1 region. This is the vibration that results from the stretching of the O-H bond of water. The broadness and the position of the band are the result of hydrogen bonding in the water molecule, either to other water molecules or to hydrogen bond acceptor sites on another molecule. If the compound is anhydrous, this region of the spectrum will be flat. The IR spectrum of Zn(acac)2∙H2O is shown in Fig. 4. 2 O-H stretch 3500 3000 2500 2000 1500 WAVENUMBERS/cm 1000 -1 Figure 4. IR spectrum of Zn(acac)2∙H2O You will also obtain a 1H NMR spectrum of your compound and compare the spectrum with that of the acetylacetone starting material. In the NMR spectrum of pure acetylacetone, you will be able to observe the keto-enol equilibrium of this compound A 1H NMR spectrum of your sample will show how the spectrum of acetylacetone is affected by the reaction with Zn(II) ion. A spectrum of acetylacetone is shown in Fig. 5 with assignments of the keto (k) and enol (e) forms. CH3 (e) DMSO acetylacetone CH3(k) H2O CH2 (k) CH (e) 7 6 5 4 3 2 1 Chemical Shift/ppm Figure 5. The 1H NMR spectrum of acetylacetone in dmso-d6. Obtained on a Varian 300 MHz NMR spectrometer. Although it is possible to identify the presence of H2O in a sample by IR spectroscopy, it is not possible to get a good quantitative measure. Another method, known as Thermogravimetric Analysis (TGA) does give a quantitative measure of the amount of H2O. In this method, a very small sample of material is placed in a platinum dish suspended from a balance. The sample is placed in an oven and slowly heated, usually 3 in an atmosphere of nitrogen. As the sample is heated it loses mass as it decomposes and this mass loss is recorded at the temperature at which it occurs. A compound that contains coordinated water will usually lose H2O around 100- 120 °C over a fairly small temperature range, while a sample that is simply wet, will lose water gradually as soon as heating is begun. Figure 6. TGA spectrum of Zn(acac)2∙xH2O In Fig. 6 the water of hydration is lost between 78-114 oC and further decomposition (loss of acac) occurs until ~250 oC. A small amount of mass is additionally lost up to ~600 oC with metallic Zn as the ultimate product. Experimental Procedure CAUTION: Acetone is a highly flammable organic solvent and must not be used or kept near an open flame. The 75% acetone solution that is used for recrystallization is also flammable. You should avoid contact with the skin or inhalation of the vapors of acetone and acetylacetone. It is advised that you wear gloves and work in a fume hood or well ventilated area. 4 Synthesis of Zn(acac)2•H2O. This portion of the experiment will require one period plus a little time during a second period5. Weigh 0.80 g of zinc oxide and place it in a beaker with 25 mL of distilled water. Heat the mixture to boiling and then remove it from the heat. Add 3 mL of acetylacetone dropwise with continuous stirring to the ZnO mixture. What changes did you observe? When the addition is complete, place the beaker in an ice bath until its contents are thoroughly cooled. Suction filter the precipitate on a small Buchner funnel with filter paper. Wash the precipitate with two or three portions of cold water and suck out as much of the water as possible. The solid that has been obtained is probably a mixture of Zn(C5H7O2)2.H2O, Zn(C5H7O2)2.2H2O, and unreacted ZnO. It is necessary to recrystallize it in order to obtain pure Zn(C5H7O2)2.H2O. To do this, prepare a solution of acetone and distilled water that contains 35 mL of acetone plus 15 mL of water and add 1 mL of acetylacetone to it. The acetylacetone serves to prevent decomposition of the product. Finally, add the precipitate to the solution and stir thoroughly. What is the appearance of the solution? Fit a long- or short-stem funnel with a piece of fine filter paper for a gravity filtration and filter the mixture. The filtrate, which should be clear but not necessarily colorless, is collected in a beaker. Cover the beaker with a watch glass or a loosely fitting piece of aluminum foil and store it as directed by your instructor until the next period. It is desirable that partial evaporation of the solvent occur during the interim. It is likely that crystals will have formed during the time in which the beaker of solution sat between periods. The procedure for the next step depends on whether there is still solvent in the beaker (Step (a)), or whether the solvent is all evaporated (Step (b)). Step (a). If there is still solvent present, cool the crystals and remaining solution by placing the beaker in an ice bath and, concurrently, cool about 15-20 mL of acetone to be used as a wash solvent. Suction filter the crystals and wash with two small portions (5 and 10 mL) of cold acetone to remove impurities, mainly the acetylacetone that was added during the recrystallization. Step (b). If all of the solvent has evaporated, transfer the crystals to the filter funnel and wash with three small portions of cold acetone. In either case, the product should be air-dried. When it is thoroughly dry, place it in a tared sample bottle and weigh it to obtain the mass of your product. Record this on the report sheet. Label the bottle with your name and the formula of the product. Melting Point. Determine the melting point and record it on the report sheet. Infrared Spectrum. Obtain an infrared spectrum of your Zn complex as a solid. TGA Analysis. In order to run a TGA analysis requires a considerable amount of time. Therefore, it is likely that only one or two samples from the class can be run. If it is not 5 possible to obtain the analysis of your sample, your instructor will give you a plot to analyze or instruct you to use Fig. 5. 1H NMR Spectra. Obtain two clean 5 mm NMR tubes from your instructor. Weigh approximately 5 mg of your dry Zn(acac)2 product into a small vial or small test tube and add 0.75 mL of DMSO-d6. Carefully swirl the liquid to dissolve your sample; it should dissolve completely. Transfer the solution to one of the NMR tubes and stopper it. In another NMR tube place 2 drops of pure acetylacetone and 0.75 mL of DMSO-d6, stopper it and invert several times to mix the two liquids thoroughly. See your instructor for directions on how to obtain a 1H NMR spectrum and do so for each of your samples. Set the resonance of the residual DMSO protons at 2.50 ppm as your internal reference. Integrate each line in each spectrum except the DMSO resonance (a multiplet) at 2.50 ppm and a H2O resonance which you may observe at about 3.3 ppm. NMR Data Treatment. Assign each of the resonances in the spectra to a specific proton in the compound. From the integrated intensities of the NMR lines in the spectrum of pure acetylacetone determine the mole ratio of the two tautomers. Which tautomer is more stable under the conditions at which you ran your spectrum? Examine the spectrum of your product. The presence of a resonance for H2O may not necessarily indicate that the compound is a hydrate because DMSO absorbs H 2O from the air very easily or it could be that the sample was not completely dried of the recrystallization solvent. References 1. Wikimedia Commons: public domain. 2. Bennett, M. J.; Cotton, F. A.; Eiss, R. Acta Cryst. 1968, B24, 904-913. 3. Lippert, E. L.; Truter, M. R. J. Chem. Soc. 1960, 4996-5006. 4. Montgomery, H.; Lingafelter, E. C. Acta Cryst. 1963, 16, 748-752. 5. Adapted from Walmsley, J. A.; Walmsley, F. Chemical Principles, Properties, and Reactions in the Laboratory, Addison-Wesley: Reading, MA, 1985, pp. 180-182. 6 Report 1. Mass of Zn-acac compound obtained ________________________ 2. Melting point of your product _______________________ 3. Analysis of 1H NMR spectra. A. Acetylacetone (a) Keto form: Chemical shift of CH3 (in ppm) _____________ Integration of CH3 _______________________ Chemical shift of CH2 _____________________ (b) Enol form: Chemical shift of CH3______________________ Integration of CH3 ________________________ Chemical shift of CH ______________________ Chemical shift of OH ______________________ (c) Mole ratio of keto to enol form of acetylacetone in your sample. Show your calculations. B. Zn-acac Complex (a) Chemical shift of CH3 ______________________ Chemical shift of CH _______________________ Chemical shift of H2O ___________________ 7 (b) Suppose that the acac was bonded as a monodentate ligand to the Zn(II) ion as shown here. How would the 1H nmr spectrum be different from your spectrum? 4. Examine your IR spectrum. (a) If present, what is the frequency of the O-H stretching vibration in cm-1?__________________ (b) Does your product contain H2O? 5. (a) Calculate the percent mass loss corresponding to the loss of H2O (between ~ 80-120 oC). See Fig. 5._________________ (b) Calculate the number of moles of H2O that this mass loss corresponds to. ____________ 8 6. Taking into account all of the data you have collected, what do you conclude is the likely formula of your product? 7. Calculate the theoretical yield of your compound. 8. What is the percentage yield? 9. Attach your IR spectrum and your 1H NMR spectra to this report. Be sure to put your name on each spectrum. 9