CeO2 is a reducible oxide having fluorite structure

Structure of Ce
1–x
Sn
x
O
2
and its relations to oxygen storage property from first-principles analysis
Supplementary Information
Asha Gupta
1
, Anil Kumar
2
, M. S. Hegde
3
and U. V. Waghmare
2
*
1
Materials Research Centre, Indian Institute of Science, Bangalore 560012, India
2
Theoretical Sciences Unit, Jawaharlal Nehru Centre for Advanced Scientific Research,
Bangalore 560064, India
3 Solid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore 560012, India.
* Corresponding author’s email: waghmare@jncasr.ac.in
Experimental Techniques
CeO
2
, SnO
2
, Ce
0.75
Sn
0.25
O
2
and Ce
0.5
Sn
0.5
O
2
have been prepared by solution combustion method.
1
SnC
2
O
4
is used as a precursor for SnO
2
; and is synthesized by dissolving SnCl
2
in acidic water giving clear solution followed by precipitation of SnC
2
O
4
crystals by slowing adding saturated oxalic acid solution. A typical combustion reaction for preparation of SnO
2
, CeO
2
and
Ce
1– x
Sn x
O
2
can be written as follows:
9(1– x )(NH
4
)
2
Ce(NO
3
)
6
+ 9 x Sn(NO
3
)
4
+ 4(6– x )C
2
H
5
NO
2
9Ce
1– x
Sn x
O
2
+ 8(6– x )CO
2
+
(96–46 x )H
2
O + (48–26 x ) N
2
For example, preparation of Ce
0.5
Sn
0.5
O
2
was carried out by taking 5.48 g ( 0.01 mol ) of
(NH
4
)
2
Ce(NO
3
)
6
(Loba Chemie, 99% ), 2.07 g (0.01 mol ) of SnC
2
O
4
(precipitated from SnCl
2
,
Sigma Aldrich, 99.9%), and 3.67 g (0.049 mol ) of glycine (C
2
H
5
O
2
N, Merck, 99%) in a 300 mL crystallizing dish, and dissolved in minimum volume of HNO
3
and 20 mL of water to form a clear solution. The dish was then kept in a pre-heated furnace at 320 o C . The combustion started after dehydration, and the product was obtained within 60 seconds.
1
The powder XRD studies of the product were recorded on Philips X’Pert diffractometer at a scan rate of 0.12
o min –1 with 0.02
o step size in the 2θ range between 20 and 100 degrees. The
Rietveld refinement of the powder XRD patterns were carried out using FullProf–fp2k program
2 by simultaneously varying 18 parameters that include the overall scale factor, background parameters, unit cell, half width, shape, and isotropic thermal parameters along with the oxygen occupancy.
Results and Discussions
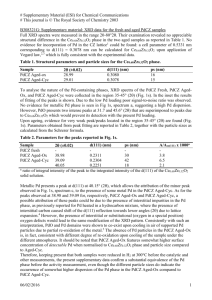
XRD : The Rietveld refinement of the powder XRD patterns of CeO
2
, SnO
2
and Ce
0.5
Sn
0.5
O
2 shows that the profile fitting is good (Figure S1). All the diffraction lines in the X–ray diffraction pattern of SnO
2
could be indexed to rutile structure (JCPDS No.
770449
), and those of CeO
2 and
Ce
0.5
Sn
0.5
O
2
could be indexed to fluorite structure (JCPDS No.
340394
). No diffraction lines corresponding to SnO or SnO
2
were observed in the powder diffraction pattern of Ce
1– x
Sn x
O
2
.
Our estimates of the lattice parameter, R f
, R
Bragg
and χ 2 values after complete refinement (see
Table 1), show that the lattice parameter of the solid solution decreases monotonously with Sn concentration ( x ), which is expected from smaller ionic radius of Sn
4+
ion (r = 0.81 Å ) than of
Ce
4+
ion (r = 0.97 Å ).
TEM : High-resolution TEM (HRTEM) image of SnO
2
and Ce
0.75
Sn
0.25
O
2
(Figure S2), and the corresponding selected area electron diffraction (SAED) pattern were recorded. Crystallite sizes for SnO
2
are in the range of 15–20 nm. HRTEM image shows lattice fringes of 3.35 Å corresponding to (110) planes of rutile-SnO
2
, and distinct lattice fringes and ring pattern indicate that the particles are highly crystalline in nature, and amorphous phase was not detected.
HRTEM image for Ce
0.75
Sn
0.25
O
2
shows lattice fringes of 3.07 Å corresponding to (111) planes of the solid solution. Crystallites sizes are within the range SAED of the solid solution show distinct
2
ring patterns for the fluorite structure and ring pattern corresponding to rutile phase were not observed. Well defined lattice fringes and diffraction pattern indicates that the particles are crystalline in nature. Therefore absence of SnO
2
phase, within our detection limits, and significant decrease in lattice parameter (from Rietveld analysis) indeed show that Sn 4+ ion are substituted for Ce
4+
forming Ce
0.75
Sn
0.25
O
2
solid solution.
3
Figure S1 . Reitveld refined XRD patterns of (a) CeO
2
, (b) SnO
2
and (c) Ce
0.5
Sn
0.5
O
2
.
4
(a)
(a)
3.35 Å
(b)
3.07
Å
Figure S2 . HRTEM of (a) SnO
2
, (b) Ce
0.75
Sn
0.25
O
2
, the inset shows the selected area diffraction
(SAED) pattern.
5
Reference
1
T. Baidya, A. Gupta, P. A. Deshpandey, G. Madras, and M. S. Hegde, J. Phys. Chem. C 113
(10), 4059 (2009).
2
J. Rodriguez-Carvajal, Multi-pattern Rietveld Refinement program Fullprof. 2k, version 3.30
June 2005-LLB
6