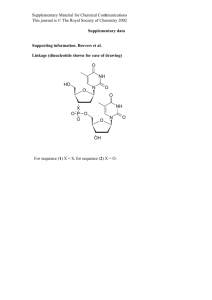
Synthesis, experimental details, and calculated spin density
advertisement
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
Supporting Information
Trityl-nitroxide biradicals as unique molecular probes for the
simultaneous measurement of redox status and oxygenation
Yangping Liu,† Frederick A. Villamena,†,‡ Antal Rockenbauer§ and Jay L. Zweier†,*
†
Center for Biomedical EPR Spectroscopy and Imaging, The Davis Heart and Lung Research Institute,
the Division of Cardiovascular Medicine, Department of Internal Medicine and ‡Department of
Pharmacology, College of Medicine, The Ohio State University, Columbus, OH, 43210, USA,
§
Chemical Research Center, Institute of Structural Chemistry, P.O. Box 17, H-1525 Budapest,
Hungary.
* Email: Jay.Zweier@osumc.edu
Supplementary Materials Table of Contents
I. EPR Spectroscopy
S2
II. Determination of J-coupling values of biradicals
S2
III. Reaction of biradicals with ascorbic acid detected by EPR S3-5
spectroscopy
IV. Reaction of biradicals with ascorbic acid detected by UV-vis S6
spectroscopy
V. Kinectic study of biradicals with ascorbic acid detected by EPR S7-10
spectroscopy
S11
VI. Oxygen sensitivity
VII. Spin density distribution on the basis of theoretical calculation at
the B3LYP/6-31+G(d,p)//HF/3-21G(d) level of theory
VIII. Synthesis
S1
S12-19
S20-22
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
I.
EPR spectroscopy
EPR spectra were recorded at room temperature using Bruker EMX X-band spectrometer
with high sensitivity resonator. The following acquisition parameters were used in the
data acquisition: modulation frequency, 100 kHz; microwave frequency, 9.85 GHz;
modulation amplitude, 0.03-0.2 G; time constant, 10-41 ms; scan time, 10-42 s; and
microwave power, 1-5 mW.
II. Determination of J-coupling values of biradicals
In order to determine the J values and the confidence intervals, a complete parameter
adjustment we carried out using an EPR simulation program (Rockenbauer, A. and
Korecz, L. (1996) Automatic computer simulations of ESR spectra. Appl. Magn. Reson.
10, 29-43). The following parameters were optimized: g1, g2, AN, the relaxation or line
width variation parameters: , , and , and J. This method gave the optimum J values of
J = 300 G and 52.5 G for TN1 and TN2, respectively. Thereafter, we systematically
changed the J value, and then fixing this value again with reoptimization of the other six
parameters and the least square errors were determined in all cases. When J was changed
the least square error increased. The noise level of the spectra was also determined as
well as for the J' and J", where we found that the least square error was larger than the
optimum value by the amount of the noise level.
Then J' and J" can be considered as the limits of the confidence interval:
TN1: J' = 160 G, J" is infinite;
TN2: J' = 52.3 and J" = 52.8 G.
Consequently the error bracket is ± 0.3 G for TN2, while for TN1 only we can state the
correct J should be larger than 160 G, but the upper limit cannot be determined.
S2
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
III. Reaction of biradicals with ascorbic acid detected by EPR
spectroscopy
Figure S1. EPR spectra obtained by reaction of TN2 (10 μM) with ascorbic acid (400 μM)
in PBS (50 mM, pH 7.4) after 45s and 10 min. Spectra were recorded with 5 mW
microwave power and 0.2 G modulation amplitude.
S3
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
3526
3525
3527
3526
3528
3527
Field
(G)
3529
3528
3530
3529
Figure S2. The partially overlapping triplet EPR signal obtained by reaction of TN1 (10
μM) with Ascorbic acid (400 μM) in PBS (50 mM, pH 7.4) after 20 min. Spectra were
recorded with 5 mW microwave power and 0.05 G modulation amplitude.
3526
3527
3528
Field (G)
3529
3530
Figure S3. The singlet EPR signal obtained by reaction of TN2 (10 μM) with Ascorbic
acid (400 μM) in PBS (50 mM, pH 7.4) after 20 min. Spectra were recorded with 5 mW
microwave power and 0.05 G modulation amplitude.
S4
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
Experimental
Simulated
3520 3522 3524 3526 3528 3530 3532 3534
Field (G)
Figure S4. Experimental and Simulated EPR spectra of the trityl-hydroxylamine TN1-H.
In the computer simulation,
13
C hyperfine splittings from three aromatic groups were
considered.
S5
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
IV. Reaction of biradicals with ascorbic acid detected by UV-vis
spectroscopy
Figure S5. UV-vis spectra of 10 μM TN1 incubated with ascorbic acid (400 μM) over a
period of 0-20 min in PBS (50 mM, pH 7.4).
Figure S6. UV-vis spectra of 10 μM TN2 incubated with ascorbic acid (400 μM) over a
period of 0-20 min in PBS (50 mM, pH 7.4).
S6
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
V. Kinectic study of biradicals with ascorbic acid detected by
EPR spectroscopy.
Various concentrations of ascorbic acid (50, 100, 200 and 400 μM) were added to the
solution of TN1 or TN2 (10 μM) in PBS (50 mM, pH 7.4). Incremental EPR spectra were
recorded 45 s after mixing and this point was considered to be t = 0 in Figure S7, S8, S10
and S11. The concentration of trityl-hydroxylamines, TN1-H or TN2-H, at each time
point was obtained by comparing their double integrated signal intensities relative to
CT03 as standard. Since the ascorbic acid concentration (50, 100, 200 and 400 μM) used
was in greater excess than the biradical concentration (10 μM), the reaction kinetics of the
biradical with ascorbic acid is a pseudo first-order reaction. The rate laws for this reaction
are shown below:
d TN
k 2 [ Asc][TN ]
dt
TN TN 0 TNH
k obs k 2 [ Asc]
d TN 0 [TNH ]
k 2 [ Asc]TN 0 [TNH ] k obs TN 0 [TNH ]
dt
d TN 0 [TNH ]
k obs TN 0 [TNH ]
dt
TN 0 [TNH ]
k obst
ln
TN 0
where [TN] is the concentration of biradical; k2 is the second-order rate constant; [Asc] is
the ascorbic acid concentration used; [TN]0 is the initial concentration of biradical; [TNH]
is the concentration of the trityl-hydroxylamine. The approximated second order rate
S7
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
constant k2 was finally calculated from the slope of the plot of kobs versus [Asc]. The
standard deviation for k2 was obtained from the various points shown in plots S9 and S12.
Figure S7. Trityl signal formation in the reaction of TN1 (10 μM) with various
concentrations of ascorbic acid in PBS (50 mM, pH 7.4).
Figure S8. Plot of ln{([TN]0-[TNH])/[TN]0} vs t for TN1
S8
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
Figure S9. Plot of kobs vs [Asc] for TN1
Figure S10. Trityl signal formation in the reaction of TN2 with various concentrations of
ascorbic acid in PBS (50 mM, pH 7.4).
S9
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
Figure S11. Plot of ln{([TN]0-[TNH])/[TN]0} vs t for TN2
Figure S12. Plot of kobs vs [Asc] for TN2
S10
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
VI. Oxygen sensitivity
In order to measure oxygen sensitivity of the trityl-hydroxylamine TN1-H and
TN2-H, ascorbic acid (2 mM) was added to a solution of biradicals (100 μM) in PBS
buffer. After 10 min, the solution was transferred into a gas-permeable Teflon tube (i.d. =
0.8 mm) and was sealed at both ends. The sealed sample was placed inside a quartz EPR
tube with open ends. Nitrogen or N2/O2 gas mixture with varying concentrations of O2
was allowed to bleed into the EPR tube and after about 4 min, was changed into another
gas mixture. EPR spectra were recorded using a model of incremental sweep. According
to the resulting spectra, the peak-peak linewidth and the spectral ratio (Iin/Iout) were
calculated.
Figure S13. Plot of the spectral ratio (Iin/Iout) as a function of time.
S11
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
VII. Spin density distribution on the basis of theoretical
calculation at the B3LYP/6-31+G(d,p)//HF/3-21G(d) level of
theory
Figure S14. Optimized structure of TN1
S12
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
Table S1. Charge and spin density distribution of TN1.
atom
C
C
C
C
C
C
C
S
S
S
S
C
C
C
C
C
C
C
C
C
C
C
C
C
C
C
C
C
C
S
S
C
C
C
S
S
C
C
C
S
S
C
C
C
S
S
C
atom #
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
charge
-0.13266
-0.2045
-0.155
3.05239
-0.11438
-0.20304
0.10285
0.38701
0.36374
0.40825
0.35359
-0.39471
-0.39291
-0.69693
-0.70118
-0.69857
-0.69655
-0.12757
-0.18818
-0.18164
-0.15066
-0.14972
-0.18436
-0.10077
-0.19615
-0.13382
-0.20265
-0.16226
-0.19398
0.39094
0.35382
-0.39023
-0.69906
-0.69778
0.31822
0.36019
-0.37286
-0.70005
-0.69805
0.34672
0.37816
-0.38762
-0.69694
-0.69946
0.35235
0.40121
-0.39244
S13
spin density
-0.02788
0.0638
-0.0286
0.0793
-0.06782
0.0746
0.61417
-0.00373
0.0163
-0.00217
0.00711
-0.00039
-0.00075
1E-05
0.00042
0.00019
-1E-05
-0.07175
0.08264
-0.03282
0.07694
-0.0316
0.09012
-0.06901
0.08081
-0.02702
0.06347
-0.0278
0.07722
-0.00328
0.01118
-0.00027
0.0002
-4E-05
-0.0036
0.00697
0.0002
1E-05
-1E-05
0.00682
-0.0027
-0.0002
-2E-05
0.00012
0.00811
-0.00181
-0.00038
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
C
C
C
O
O
C
O
C
O
O
C
C
C
C
C
C
C
C
C
N
O
N
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
96
97
-0.69768
-0.69901
0.80283
-0.59479
-0.72674
0.68434
-0.6214
0.80604
-0.58995
-0.72735
-0.08201
-0.47339
-0.47382
0.09457
0.09761
-0.6845
-0.69278
-0.68373
-0.69181
-0.03971
-0.42358
-0.64929
0.25179
0.25136
0.25947
0.24437
0.25954
0.24822
0.24357
0.25393
0.24616
0.26007
0.24824
0.25231
0.24587
0.24335
0.25318
0.2484
0.25178
0.26389
0.25765
0.24575
0.24915
0.25429
0.25115
0.26205
0.25176
0.25985
0.2508
0.2569
S14
-4E-05
0.00031
-0.00043
0.01609
0.00202
-0.00268
0.00892
-0.00046
0.01451
0.00183
1E-05
0.00127
0.00102
-0.01573
-0.01599
0.00878
0.0268
0.00869
0.02685
0.42865
0.536
0.00325
-1E-05
0
-1E-05
-1E-05
-2E-05
-1E-04
-9E-05
-1E-05
0.0001
-1E-05
0
-5E-05
0.00015
-7E-05
-2E-05
0
-6E-05
-1E-05
-1E-05
0.00013
-3E-05
1E-05
-5E-05
-1E-05
-4E-05
-1E-05
0
-2E-05
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
98
99
100
101
102
103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
119
120
121
122
123
124
125
0.24538
0.24524
0.24911
0.25184
0.26406
0.24643
0.24506
0.25336
0.53181
0.5332
0.26267
0.24603
0.27444
0.24639
0.25645
0.24395
0.23756
0.26939
0.23589
0.26499
0.24213
0.24257
0.26671
0.24035
0.24791
0.26078
0.2356
0.43556
S15
1E-04
-6E-05
-1E-05
-6E-05
0
7E-05
-8E-05
0
-5E-05
-0.00016
3E-05
5E-05
-0.00066
0.00011
-0.00065
0.00013
-0.00104
-0.00039
-0.00083
-0.00101
0.00063
0.00013
-0.00037
-0.00103
0.00063
-0.00106
-0.00084
-8E-05
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
Figure S15. Optimized structure of TN2.
S16
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
Table S2. Charge and spin density distribution of TN2
C
C
C
C
C
C
C
S
S
S
S
C
C
C
C
C
C
C
C
C
C
C
C
C
C
C
C
C
C
S
S
C
C
C
S
S
C
C
C
S
S
C
C
C
S
S
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
charge
-0.13569
-0.20075
-0.15708
-0.19538
-0.11752
-0.20126
0.09813
0.38435
0.36088
0.40803
0.36324
-0.39075
-0.39071
-0.70161
-0.6974
-0.70127
-0.6963
-0.10162
-0.19472
-0.15442
-0.20137
-0.127
-0.20684
-0.09057
-0.18916
-0.16867
-0.19918
-0.13871
-0.20173
0.36211
0.36119
-0.38898
-0.70161
-0.69679
0.40791
0.35874
-0.39221
-0.6973
-0.69941
0.35076
0.37591
-0.38783
-0.69976
-0.69767
0.34367
0.39824
S17
spin
density
-0.02697
0.06267
-0.026
0.07226
-0.06446
0.07178
0.62277
-0.00333
0.01598
-0.00309
0.01712
-0.00077
-0.00083
0.00029
0
0.00035
0
-0.06918
0.08094
-0.02784
0.06417
-0.02892
0.07312
-0.06959
0.0791
-0.02657
0.06232
-0.02727
0.07673
-0.00361
0.01593
-0.00086
0.00041
3E-05
-0.00191
0.00754
-0.00031
-2E-05
0.00023
0.00616
-0.00259
-0.00021
0.0002
-3E-05
0.00793
-0.0019
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
C
C
C
C
O
O
C
O
C
O
O
O
C
C
N
C
C
C
C
C
O
C
C
C
C
N
O
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
47
48
49
50
51
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
96
-0.38959
-0.69934
-0.69737
0.80643
-0.58984
-0.72555
0.81376
-0.58915
0.80637
-0.59118
-0.72613
-0.57905
-0.19233
0.65547
-0.65663
-0.08384
-0.47241
-0.47258
0.09436
0.0968
-0.6429
-0.69258
-0.68341
-0.69186
-0.68379
-0.03964
-0.42336
0.24848
0.2597
0.24512
0.25976
0.25146
0.25243
0.24558
0.25932
0.24725
0.25394
0.26121
0.24592
0.26024
0.24507
0.25113
0.25252
0.25771
0.25362
0.26006
0.25284
0.25172
0.25363
0.24664
S18
-0.00031
0.00032
-3E-05
9E-05
0.01464
0.00185
-0.00088
0.01531
-0.00059
0.01398
0.00181
0.00251
-0.00021
0.00019
0.00019
-6E-05
0.00103
0.00104
-0.01568
-0.01576
0.0001
0.02678
0.00863
0.02684
0.00855
0.42792
0.5366
-8E-05
-2E-05
0
0
0
-1E-05
-8E-05
-2E-05
-1E-05
0
-1E-05
0
-2E-05
-1E-05
-9E-05
0
-1E-05
0
0
-4E-05
0
-1E-05
-8E-05
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
97
98
99
100
101
102
103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
119
120
121
122
123
124
125
126
127
128
129
130
131
0.24686
0.24978
0.24583
0.25349
0.25969
0.2516
0.2542
0.24726
0.25422
0.24611
0.2535
0.25046
0.25952
0.53207
0.53282
0.2601
0.27515
0.44208
0.26531
0.24169
0.27198
0.24491
0.25782
0.26481
0.24213
0.23623
0.23491
0.26949
0.24413
0.24753
0.26127
0.2361
0.26734
0.23997
0.24195
S19
1E-04
-6E-05
7E-05
1E-05
-1E-05
-4E-05
0
8E-05
0
-7E-05
-6E-05
0
0
-5E-05
-8E-05
0.00014
-1E-05
0
3E-05
7E-05
-0.00066
0.00011
-0.00066
-0.00101
0.00065
-0.00083
-0.00105
-0.00039
0.00013
0.00065
-0.00105
-0.00084
-0.00038
-0.00103
0.00015
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
VIII. Synthesis
The TAM radical CAT-03 were synthesized according to the previous procedure (Liu, Y. P.;
Villamena, F. A.; Sun, J.; Xu, Y.; Dhimitruka, I.; Zweier, J. L. J. Org. Chem. 2008, 73, 1490).
TN1
To the solution of CAT-03 (120 mg, 0.12 mmol), 4-amino-TEMPO (21 mg, 0.12
mmol), HOBt (24 mg, 0.18 mmol) and Et3N (50 μL) in dry DMF (10 ml) was added BOP
(80 mg, 0.18 mmol). The reaction mixture was stirred under N2 at room temperature for
18 h. Solvent was removed under vacuum, and the residue was dissolved in phosphate
buffer (0.2 M, pH 7.4) and purified by column chromatography on reverse phase C-18
and water followed by 0-20% acetonitrile in water as eluants to give the biradical as a
green solid (118 mg, 86 %). MS (M+, m/z): 1152.0817 (measured), 1152.0787
(calculated).
TN2
To the solution of CAT-03 (82 mg, 0.082 mmol) and 4-(2-Bromoacetamido)-TEMPO (29
mg, 0.098 mmol) in DMSO (10 mL) was added solid K2CO3 (23 mg, 0.164 mmol). The
reaction mixture was stirred for 18 h at room temperature. Then, 50 mL of the citric acid
solution (8%, w/v) and 40 mL of ethyl acetate were added and the organic layer was
separated. The aqueous solution was extracted with ethyl acetate (2x40 mL). The
combined organic solution was washed with saturated NaCl (3x50 mL) and water (1x50
mL), dried over anhydrous Na2SO4 and evaporated in vacuo. The residue was dissolved
in phosphate buffer (200 mM, pH 7.4) and purified by column chromatography on
reverse phase C-18 and water followed by 0-40% acetonitrile in water as eluants to give
S20
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
the compound as a brown solid (38 mg, 38%). MS ([M+H]+, m/z): 1211.060 (measured),
1121.092 (calculated).
Figure S16. High Resolution Mass Spectrum of TN1.
S21
Supplementary Material (ESI) for Chemical Communications
This journal is (c) The Royal Society of Chemistry 2009
Figure S17. High Resolution Mass Spectrum of TN2.
S22