The Schrödinger Equation for one particle, mass m, in 3D:
advertisement
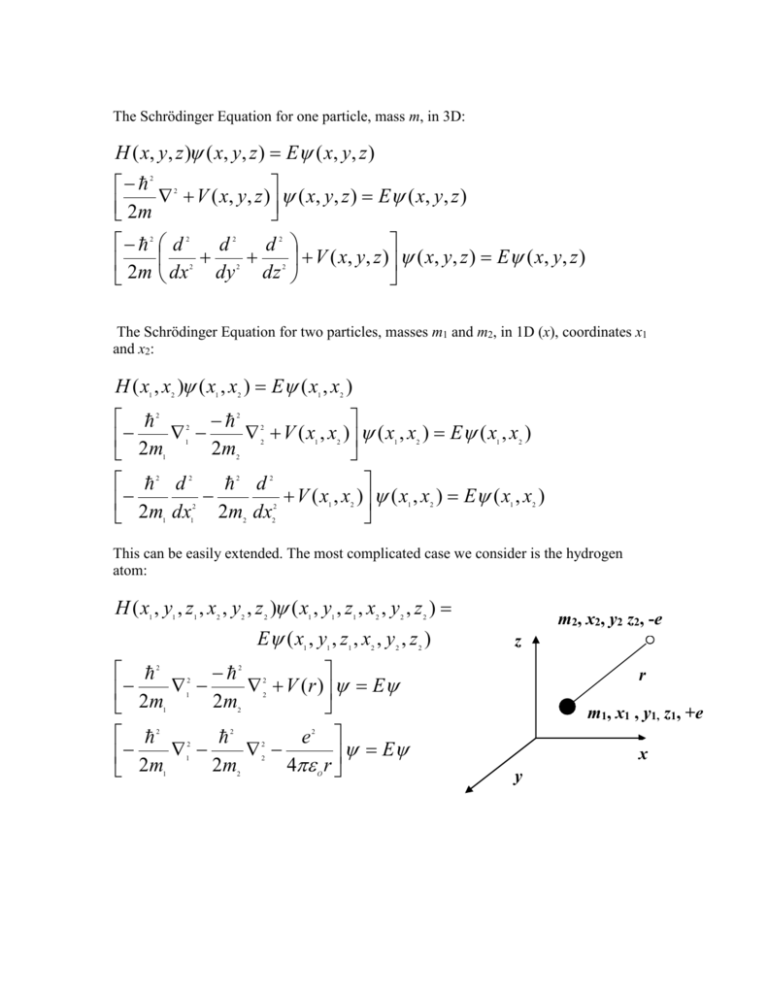
The Schrödinger Equation for one particle, mass m, in 3D: H ( x, y, z ) ( x, y, z ) E ( x, y, z ) 2m 2m 2 2 V ( x, y, z ) ( x, y, z ) E ( x, y, z ) d d d V ( x, y, z ) ( x, y, z ) E ( x, y, z ) dx dy dz 2 2 2 2 2 2 2 The Schrödinger Equation for two particles, masses m1 and m2, in 1D (x), coordinates x1 and x2: H ( x , x ) ( x , x ) E ( x , x ) 1 2 1 2 1 2 V ( x , x ) 2m ( x , x ) E ( x , x ) 2 m d d V ( x , x ) 2m dx 2m dx ( x , x ) E ( x , x ) 2 2 2 2 1 2 1 1 2 1 2 1 2 2 2 2 2 2 2 1 1 2 1 2 2 1 2 1 2 2 This can be easily extended. The most complicated case we consider is the hydrogen atom: H ( x , y , z , x , y , z ) ( x , y , z , x , y , z ) 1 1 1 2 2 2 1 1 1 2 2 E ( x , y , z , x , y , z ) 1 1 1 2 2 V ( r ) E 2m 2m e 2m 2m 4 r E 2 m2, x2, y2 z2, -e 2 2 z 2 2 2 1 2 1 m1, x1 , y1, z1, +e 2 2 2 2 2 2 1 1 r x 2 2 O y 2m r x 2 1 2 d d d dx dy dz 2m y z 2 2 2 2 2 2 1 1 1 2 2 2 2 d d d e dx dy dz 4 2 2 2 2 2 2 2 2 2 2 O E r 1/ 2 Normalization in 3D: ( x, y, z ) d ( x, y, z ) * ( x, y, z ) dx dy dz 1 2 all space d dx dy dz For the 3 types of molecular motion the energy equations obtained from solving these higher dimensional problems are: Translation in 3D – ‘Particle in the 3D box’ – energy equation above. Rotation. Rotation of a rigid 3D shape. Linear Molecules have the same energy equation as diatomic molecules. ‘Rigid Rotator.’ Energy expression for rotation of a non-linear molecule is complicated but is exactly known. The energy equation depends on the shape of the molecule. Generally there are 3 axes of rotation through the centre of mass (X, Y, Z), 3 moments of inertia about these axes, IA, IB, IC , 3 rotational constants A, B, C and 3 quantum numbers J, KA, KC . Vibrations. Polyatomic molecules have many vibrations. Many of these behave like SHO, stretches and angle bends. (Sometimes they are degenerate, SHO in 2D, e.g. the bend of CO2.) Others require different models, e.g. internal rotation of a CH3- group which behaves like a 2D rotator. Many of these problems involve changing the coordinates from Cartesian (x, y, z) to some other set of 3D coordinates. This is in order to ‘separate’ the coordinates into Schrödinger Equations in 1 dimension. Example of separating the coordinates: Particle in 3D box Particle (molecule) mass m in a 3 dimensional volume (room) of lengths A, B and C in the x, y, z directions. V(x, y, z) = ∞ if x < 0 or x >A, y < 0 or y > B or z < 0 or z > C V(x, y, z) = 0 if 0 ≤ x ≤ A and 0≤y ≤ B and 0 ≤ z ≤ C z C A B x y Now show that this 1 particle in 3D problem separates into 3, 1 particle 1D problems. The fundamental point in this example is: If the Hamiltonian can be separated: H ( x, y, z ) H ( x) H ( y) H ( z ) 1 2 3 then the one dimensional Schrödinger Equations can be solved the energies added and the wavefunctions multiplied. EE E E x y *see demonstration in 2D below z ( x, y, z ) ( x) ( y ) ( z ) 1 2 3 In this case V ( x, y, z ) y z 2m x H ( x, y, z ) 2 2 2 2 2 2 2 and clearly: V ( x, y, z ) V ( x) V ( y ) V ( z ) x 2m 2 H ( x, y, z ) therefore: 2m 2 2 y z 2 V ( x) V ( y ) V ( z ) 2 2 2 V ( x) V ( y ) V ( z ) x 2m y 2m z H ( x) H ( y ) H ( z ) 2 2 2 2 2 2 2 2 2 d H ( x) V ( x) etc., which is the 1D particle in the box in 2m dx the x direction. 2 2 where: 1 2 nh 2 n x E , ( x) sin , n 1,2,3 A A 8mA 2 Therefore: 1/ 2 2 x n 2 ph 2 p y E , ( y ) sin , p 1,2,3 B B 8mB qh 2 q z E , ( z ) sin , q 1,2,3 C C 8mC 2 and similarly: 1/ 2 2 y p 2 2 1/ 2 2 z q 2 nh ph qh E 8mA 8mB 8mC 2 2 2 2 Therefore: 2 2 2 1/ 2 *demonstration for 2 D H ( x, y ) ( x, y ) E ( x, y ) if : H ( x, y ) H ( x) H ( y ) let : ( x, y ) ( x) ( y ) and E E E 1 2 1 2 ( y ) H ( x) ( x) ( x) H ( y ) ( y ) E ( x) ( y ) throughout by ( x) ( y ) on the left H ( x) ( x) H ( y ) ( y ) EE E ( x) ( y) H ( x) ( x) E ( x) and H ( y ) ( y ) E ( y ) 1 1 1 2 1 2 2 1 2 1 1 2 2 1 2 8 n x p x q x , ( x, y, z ) sin sin sin ABC A B C n 1,2,3 , p 1,2,3 , q 1,2,3 NOTE: 1 quantum number for each coordinate! 2 2 1 1 2 2 2