PHYS-4420 THERMODYNAMICS & STATISTICAL MECHANICS
advertisement
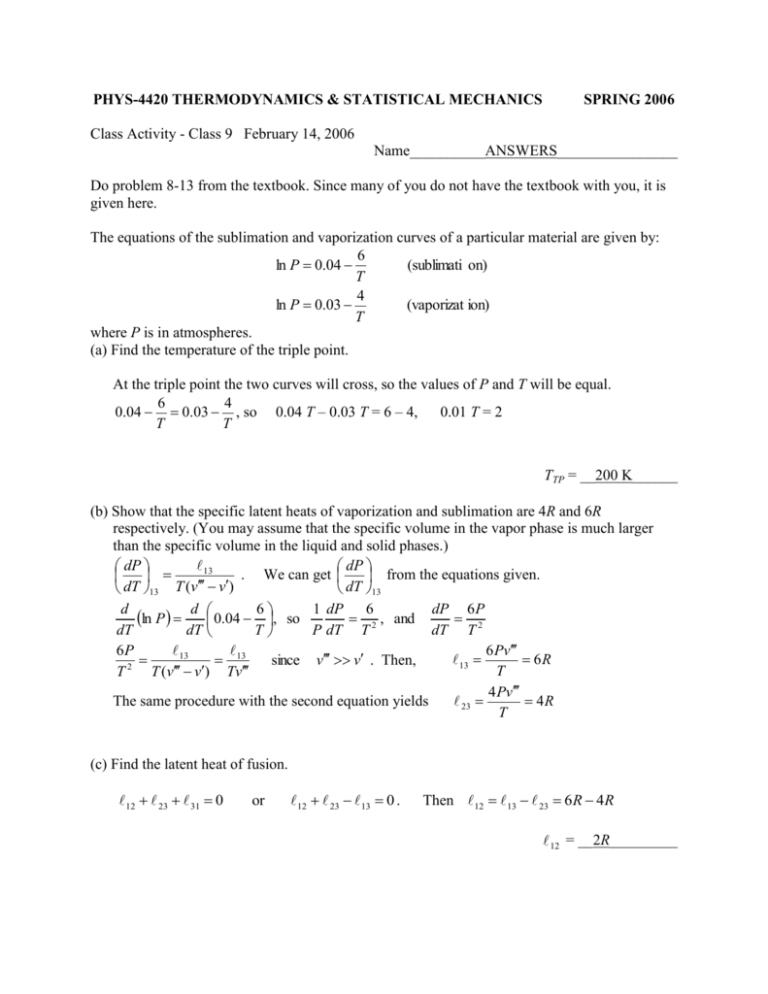
PHYS-4420 THERMODYNAMICS & STATISTICAL MECHANICS SPRING 2006 Class Activity - Class 9 February 14, 2006 Name__________ANSWERS________________ Do problem 8-13 from the textbook. Since many of you do not have the textbook with you, it is given here. The equations of the sublimation and vaporization curves of a particular material are given by: 6 ln P 0.04 (sublimati on) T 4 ln P 0.03 (vaporizat ion) T where P is in atmospheres. (a) Find the temperature of the triple point. At the triple point the two curves will cross, so the values of P and T will be equal. 6 4 0.04 0.03 , so 0.04 T – 0.03 T = 6 – 4, 0.01 T = 2 T T TTP = __200 K______ (b) Show that the specific latent heats of vaporization and sublimation are 4R and 6R respectively. (You may assume that the specific volume in the vapor phase is much larger than the specific volume in the liquid and solid phases.) 13 dP dP . We can get from the equations given. dT 13 T (v v) dT 13 d ln P d 0.04 6 , so 1 dP 62 , and dP 6 P2 dT dT T P dT T dT T 6 Pv 6P 13 13 6R 13 since v v . Then, 2 T T T (v v) Tv 4 Pv 23 4R The same procedure with the second equation yields T (c) Find the latent heat of fusion. 12 23 31 0 or 12 23 13 0 . Then 12 13 23 6R 4R 12 = __2R_________