P21 Homework Set #12..
advertisement

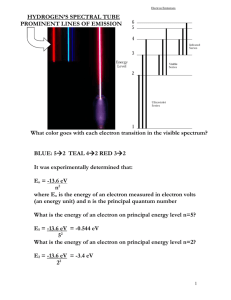
P21 Homework Set #12 Solutions Chapter 27-30 P27.17. Prepare: The earth is in reference frame S and the cosmic ray is in reference frame S. Frame S travels with velocity v relative to frame S. Two events are “cosmic ray enters the atmosphere” and “cosmic ray hits the ground.” These can both be measured with a single clock in the cosmic ray’s frame, frame S, so the time interval between them in S is the proper time interval: t . Solve: The time interval measured in the earth’s frame, frame S, is t 400 s. The time-dilation result, Equation 27.5, is 1 2 t. The cosmic ray’s speed in frame S is simply v L 60,000 m 1.5 108 m/s t 400 106 s v 0.50 c Thus the time interval measured by the cosmic ray is 1 (0.50) 2 (400 s) 350 s P27.21. Prepare: The ground is frame S and the moving rod is frame S. The length of the rod in S is the proper length l because the rod is at rest in S. The length is contracted to 60% when L 0.60 l. Solve: Using Equation 27.11, the rod is length contracted in S to L 1 2 l Using L/l = 0.60, 1 2 1 v 2 / c 2 0.60 v c 1 (0.60) 2 0.80c Assess: Because of significant change in length, we expected a high speed for the moving rod. P28.7. Prepare: Light of frequency f consists of discrete quanta, each of energy E hf hc/. The minimum energy required to free an electron is called the work function of the metal. An electron that has just absorbed a quantum of light energy can escape from the metal if its energy exceeds the work function. Solve: The lowest photon energy that creates photoelectrons from the metal is E hc (6.63 1034 J s)(3.0 108 m/s) 1eV 3.20 eV 388 109 m 1.6 1019 J This is the threshold energy. From Equation 28.4, the work function of the metal is E0 3.20 eV. Assess: Electrons are able to absorb light quanta in the photoelectric effect. P28.11. Prepare: We know the energy of a photon of frequency f and wavelength is E hf hc/ . Solve: (a) The longest wavelength is the photon with the minimum energy; longer wavelengths would not have enough energy to eject a photon. Solve the equation for . hc (6 63 1034 J s)(3 00 108 m/s) 1 eV 2 9 107 m 290 nm 19 E 4 3 eV 1 60 10 J (b) Review Example 28.4. If 4.3 eV of the energy is used just to get the electron ejected, the remaining 0.4 eV will become the kinetic energy of the electron, that is, Kmax 04 eV. vmax 2 K max 2(0 4 eV) 1 60 10 19 J 5 4 10 m/s m 9 11 1031 kg 1 eV Assess: Our data is a little different from that in Example 28.4, so we don’t expect to get exactly the same answer; however we do get an answer that is in the same ballpark, so ours is probably correct. P29.9. Prepare: Please refer to Figure P29.8. To conserve energy, the absorption spectrum must have exactly the energy gained by the atom in the quantum jumps. Solve: (a) An electron with a kinetic energy of 2.0 eV can collide with an atom in the n 1 state and raise its energy to the n 2 state. This is possible because E2 – E1 1.5 eV is less than 2.0 eV. On the other hand, the atom cannot be excited to the n 3 state. (b) The atom will absorb 1.5 eV of energy from the incoming electron, leaving the electron with 0.50 eV of kinetic energy. P29.19. Prepare: We will use Equation 29.2 or 29.18 with 0 = 91.18 nm. Solve: Photons emitted from the n 4 state start in energy level n 4 and undergo a quantum jump to a lower energy level with m < 4. The possibilities are 4 1, 4 2, and 4 3. According to Equation 29.18, the transition 4 m emits a photon of wavelength 0 (1/ m 2 1/ n 2 ) 91.18 nm (1/ m 2 1/16) These values are given in the following table. Transition 41 42 43 Wavelength 97.3 nm 486 nm 1876 nm Assess: Transitions are in the ultraviolet, visible, and infrared regions. P30.25. Prepare: The number of radioactive atoms decreases exponentially with time according to Equation 30.11. Solve: (a) The number of remaining 133B nuclei at time t is 1 N N0 2 t / t1/2 1 (1.0 1010 ) 2 2 y / (10.5 y) 8.8 109 (b) After 20 years, N (1.0 1010)(1/2)20 y/(10.5 y) 2.7 109. (c) After 200 years, N (1.0 1010)(1/2)200 y/(10.5 y) 1.8 104. Assess: Do not think that if half the nuclei decay during one half-life, then all will decay in two half-lives. P30.27. Prepare: The number of radioactive atoms decreases exponentially with time according to Equation 30.11. Solve: (a) If 90% of the sample has decayed, 10% is still present. This happens at time t such that t /t 1/2 1 1 N 0.10 N 0 N 0 2 2 ln(0.10) t t1/ 2 3.3t1/ 2 ln(0.50) t / t1/2 0.10 t 1 ln ln(0.10) t1/ 2 2 That is, 90% decay in 3.3 half-lives. (b) When 99% of the sample has decayed, 1% is left. The analysis is the same as in part (a), giving t That is, 99% decay in 6.6 half-lives. ln(0.010) t1/ 2 6.6t1/ 2 ln(0.50)