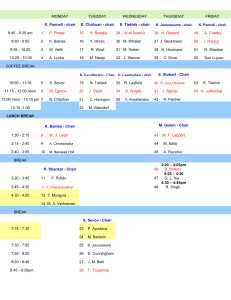
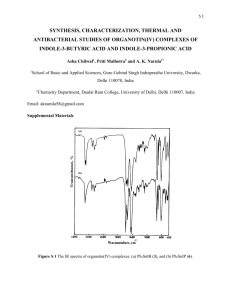
EXPERIMENT P9: Preparation and Characterisation of Organotin
advertisement

Experiment 5 Preparation and Characterisation of Organotin Compounds, (PhCH2)4-xSnBrx Organotin compounds- 1 EXPERIMENT 5: Preparation and Characterisation of Organotin Compounds, (PhCH2)4-xSnBrx. Aims to react metallic tin with benzylbromide under different conditions to characterise the products using multinuclear NMR spectroscopy to understand the origins of satellite spectra in NMR spectroscopy to characterise the products by mass spectrometry to gain an understanding of isotope patterns in mass spectrometry Introduction Organotin compounds find use in many applications, which include PVC stabilisers, agrochemicals, wood preservatives, and precursors for the chemical vapour deposition (CVD) of thin, electrically conducting films of SnO2. Recent work has also shown that certain organotin compounds have greater anti-tumour activity than cis-platin. Tin compounds have very interesting spectroscopic properties. Tin has two NMR active nuclei which may be observed directly, but which also manifest themselves in the 1H and 13C nmr spectra via spinspin coupling. Tin also has a total of ten stable isotopes and thus its compounds give highly distinctive isotope distribution patterns in their mass spectra. There are many preparative routes to organotin compounds, including (a) the direct reaction of an alkyl halide with metallic tin, (b) the treatment of tin halides with organolithium or Grignard reagents, and (c) the exchange reactions of organotin complexes and tin halides. RX + 4RMgX SnR4 R4-nSnXn Sn R4Sn SnX4 R4-nSnXn Sn + + + (a) 2MgX2 (b) (c) The products and yields of these reactions are highly dependent on the nature of the organic group and the reaction conditions. Experimental a) Preparation of Complex A. CARE: Benzyl bromide is a severe irritant and lachrymator – always wear gloves and handle in the fume cupboard. - Mix tin powder (6g) with a few drops of water to give a stiff paste and place in a round bottomed flask. - Add toluene (50 cm3) and carefully heat the mixture to boiling point. - Add benzyl bromide (6 cm 3) dropwise and reflux for the remainder of the laboratory session. - Allow to cool overnight and the next day take off the solvent on the rotary evaporator. - Recrystallise the crude product (which contains unreacted tin (grey/black)) by treating the residue with enough boiling ethyl acetate to dissolve most of the white solid. - Filter to remove the unreacted tin and add 40-60C petroleum ether carefully to the filtrate until a slight turbidity is observed. - Allow to stand and cool to room temperature to . - Filter off the fine, silky crystals formed and air dry. - If the product is not completely dry, remove any residual solvent in a vacuum desiccator. - Record the yield (g, %) and melting point of your product. Organotin compounds- 2 b) Preparation of Compound B. Begin this preparation as soon as the preparation of complex A is under way so that both are running together. - Place tin powder (3g) in a round bottomed flask, add water (50 cm 3) and bring to the boil. - Add benzyl bromide (3 cm3) carefully and reflux for 1.5 hours with vigorous stirring. The tin tends to form clumps, so it may be necessary to shake the flask from time to time to break these up. - Cool the flask on an ice bath for 20 minutes, filter the solids and air dry. - Extract the product from unreacted tin by suspending the solid in boiling ethyl acetate (15cm3). - Filter while still hot and remove the solvent from the filtrate on a rotary evaporator. - Add glacial acetic acid (20cm 3) to the residue and warm until the solid has dissolved. On cooling, white crystals of the product should form. - Filter off the product, wash with a little glacial acetic acid and air dry. - Place in a vacuum desiccator at the water pump to remove last traces of solvent. - Record the yield (g, %) and melting point of your product. Spectroscopic Interpretation. NMR and mass spectra of your two products may be obtained from a demonstrator on production of satisfactory samples of each. NMR: Tin has an unusually large number of naturally occurring isotopes, ten in all. The seven most abundant are 116Sn (0.1430), 117Sn (0.0761), 118Sn (0.2403), 119Sn, (0.0858), 120Sn (0.3285), 122Sn (0.0472) and 124Sn (0.0594). The numbers in parentheses are their fractional abundances. Of these, only two have non-zero nuclear spins, namely 117Sn and 119Sn. In both cases the spin, I = 1/2. The consequence of this is that in the NMR spectra of tin compounds satellite peaks are often observed. An intense central resonance arises from the molecules containing tin isotopes with zero nuclear spin (approx 84% of nuclei) while the signals of the 16% of molecules having 117Sn and 119Sn nuclei are doublets symmetrically disposed about the central resonance. These arise from spin-spin coupling between the tin nuclei and the observed nuclei (in this case 1H or 13C). This is illustrated in figure 1. Figure 1: 1H NMR Spectrum Showing 117/119Sn Satellites. J(117Sn - 1H) J(119Sn - 1H) Interpret your spectra as fully as possible, assigning each of the resonances. For the 1H and 13C resonances arising from the CH2 group in product B calculate the four coupling constants, Organotin compounds- 3 J(117Sn - 1H), J(119Sn - 1H), J(117Sn Hz for 1H and 100.63 Hz for 13C). 13C) and J(119Sn - 13C) in hertz. (NB 1 ppm = 400.14 Also calculate the coupling constants from the 1H spectrum of product A and compare with those in product B. Mass Spectra: In the mass spectrometer the sample is vapourised in a very high vacuum and bombarded - usually with a beam of electrons. This bombardment causes ionisation of the molecule to give a molecular ion and causes fragmentation to give fragment ions. These ions have different mass/charge ratios (m/e) and are normally separated by a strong magnetic field, detected and a signal displayed. The molecular masses obtained by this technique are very accurate (often within 10 ppm). From a knowledge of the molecular and fragment ions it is often possible to determine the molecular weight of the species and also to identify some of the structural units. In addition, elements having more than one isotope show complex isotope patterns in their molecular and fragment ions, which can be diagnostic of the species present. Shown below are the typical intensity patterns for SnBr and SnBr2 units. SnBr 2 272 274 276 278 280 Mass Number 282 284 286 SnBr 192 194 196 198 200 202 Mass Number 204 206 Interpret your mass spectra as fully as you can. Determine the molecular weight of the parent ion and try to identify the more prominent fragment ions. Which of the above isotope patterns most closely correlates to those observed in each of your spectra. Using all the information available, propose structures for both of your compounds. Organotin compounds- 4