Photoinduced Magnetization in RbCo[Fe(CN)6]
advertisement
![Photoinduced Magnetization in RbCo[Fe(CN)6]](http://s3.studylib.net/store/data/005886955_1-3379688f2eabadadc881fdb997e719b1-768x994.png)
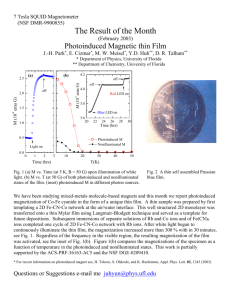
7 Tesla SQUID Magnetometer (NSF DMR-0113714) The Result of the Month (November 2002) Photoinduced Magnetization in RbCo[Fe(CN)6] J.-H. Park*, E. Cizmar*, M. W. Meisel*, Y.-D. Huh**, C. Liu**, D. R. Talham** * Department of Physics, University of Florida ** Department of Chemistry, University of Florida 2.0 2.0 M (10 emu G / sample) 1.5 1.6 1.0 0.5 Fe Co C N Light ON 1.4 ON 1.2 ON Rb 0.0 0 50 100 150 200 250 OFF OFF -5 M (emu G / mol) H = 25 G T=5K 1.8 1.0 300 0 2 Fig. 1. Field-Cooled M vs T measured with H = 25 G without the light. As temperature rises, Magnetization is decreasing. The inset shows a structure of RbCo[Fe(CN)6 ]. 4 6 8 10 12 Time (hrs) T(K) Fig. 2. M vs Time upon illumination of light. The inset shows a local heating effect (see text). Photoinduced molecule-based magnets have been of recent interest [1, 2]. Here we present a preliminary result of a known photoinduced magnet, RbCo[Fe(CN)6] synthesized by C. Liu and Y.D. Huh in Dr. Talham’s group. RbCo[Fe(CN)6] is one of the Prussian blue analogues in which Fe and Co sites are located on vertices of the cubic lattice and each metallic sites are connected by six CN ligands (Fig. 1.). A sample is field-cooled (FC) to 5 K without a light, then with the light on through a homemade optical probe, the magnetization (M) was measured as a function of time (Fig. 2.). For 12 hours of illumination of light, the M increased by ~ 100 % and still had not saturated. The inset (Fig. 2.) shows a local heating effect of the sample when the light is on and off. When the light is off, the sample cools down instantaneously and shows more magnetization (see Fig. 1. for temperature dependence of M). However, M stays the constant without the light. On the other hand, when the light is on, the sample is heated and yields lower M, but irradiation keeps M increasing over the time. In this particular sample, photoinduced magnetization is believed to be due to a charge transfer from Fe2+ to Co3+, resulting a transition from Fe2+(S=0) / Co3+(S=0) to Fe3+(S=1/2) / Co2+(S=3/2). This work is partially supported by the ACS-PRF-36163-AC5 and the NSF DGE-0209410. [1] Sato et al, Inorg. Chem. 38, 4405 (1999). [2] T. Kawamoto, Y. Asai, and S. Abe, Phys. Rev. Lett. 86, 348 (2001). Questions or Suggestions e-mail me juhyun@phys.ufl.edu