doc
advertisement
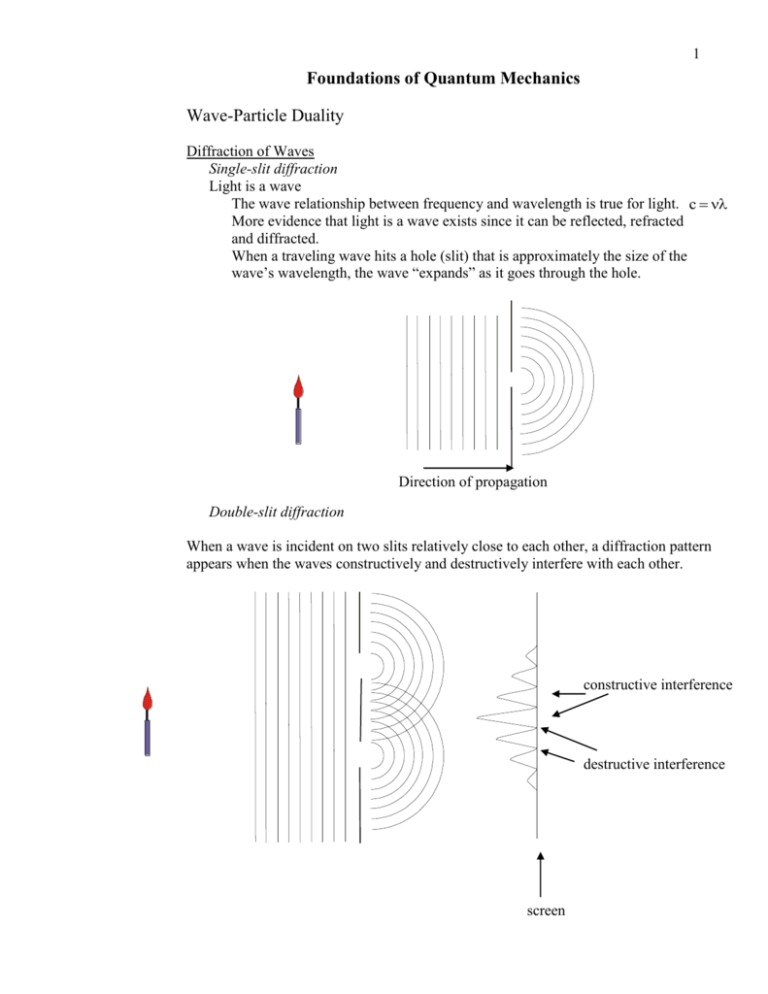
1 Foundations of Quantum Mechanics Wave-Particle Duality Diffraction of Waves Single-slit diffraction Light is a wave The wave relationship between frequency and wavelength is true for light. c More evidence that light is a wave exists since it can be reflected, refracted and diffracted. When a traveling wave hits a hole (slit) that is approximately the size of the wave’s wavelength, the wave “expands” as it goes through the hole. Direction of propagation Double-slit diffraction When a wave is incident on two slits relatively close to each other, a diffraction pattern appears when the waves constructively and destructively interfere with each other. constructive interference destructive interference screen 2 Comments about diffraction Maximum intensity is directly behind barrier between two slits. Intensity pattern comes from interference of two wavefronts. Diffraction confirms that light is a wave. Photoelectric Effect Light shining on a metal surface may cause electrons to be ejected from the surface. e- Frequency of light needs to be above threshold frequency to induce photoelectric emission. Kinetic energy of electrons is proportional to frequency of incident radiation. Kinetic energy of electrons is independent of light intensity. - I.e., microwave laser will not induce photoelectron emission. This independence contradicts wave nature of light. - According to wave nature, energy of electrons should be proportional to the intensity. **Albert Einstein proposes in 1905 that light has particle properties.** Nobel - 1921 - Each quanta of light (photon) has energy proportional to frequency. E h - h is Planck’s constant - h = 6.626 10-34 Js - Planck’s is a universal, fundamental constant of nature. If light is a particle as Einstein suggests, then the photoelectric effect is easily explained in terms of collisions between photons and electrons. 3 Compton Effect In 1922, Arthur H. Compton was investigating the nature of x-rays by shining them on targets with light nuclei such as paraffin. His intent was to measure the degree of diffraction as the x-rays went through the target. In addition to measuring the angles of diffraction, he found that the diffracted light had a increased wavelength at different angles of transmission. Graphic stolen from electrons.wikia.com Classically, light does not change its wavelength as it diffracts. He was able to find a numerical relationship between the scattered wavelength. 0 K 1 cos Compton was able to explain his equation based on first principles as long as he assume the following: 1.) Light consists as particles: E h hc 2.) The refraction of the light is actually the scattering of photons off of electrons. 3.) The conservation of momentum is valid especially if momentum is treated according to the theory of relativity with energy being a component of fourdimensional momentum. E 2 c2 p2 mo c2 2 Graphic stolen from physics-pelago.blogspot.com Thus, Compton was able to relate his numerical relationship to fundamental h constants such that 0 1 cos , where m0 is the rest mass of the m0c electron. Nobel - 1927 4 Conclusion Light has been shown to be a particle and a wave. Waves by their nature are “spread out” Particles by their nature are “localized”, i.e., in one place. How can light be spread out and in one place at the same time? WHO KNOWS? DeBroglie Waves Einstein (also in 1905!), in his special theory of relativity, demonstrated that the photon has momentum like a particle where the momentum is inversely proportional to the wavelength. p h Louis DeBroglie, in 1924, proposed that all matter has wave-like properties. (He did this in a 2-page article!) Nobel - 1929 Thus all moving particles have a wavelength. (deBroglie wavelength) h p Clinton Davisson and Lester Germer in 1927 confirm deBroglie waves by discovering that electrons of the correct energy will diffract from the surface of Ni metal. Nobel - 1937 - just like photons diffract off a grating. George P. Thomson in 1927 also confirms deBroglie waves by passing electron beam through thin sheet of Pt and finding diffraction rings. Nobel – 1937 - G. P. Thomson’s father, J. J. Thomson won Nobel (1906) for discovering electron as a particle. Heisenberg’s Uncertainty Principle In 1925, based on a relationship from classical wave theory and deBroglie’s hypothesis; Heisenberg formulated his Uncertainty Principle. Heisenberg’s uncertainty principle states that one cannot measure a particle’s position and momentum simultaneously with infinite precision. x p 2 where x is the uncertainty in the measurement of the particle’s position, p is the uncertainty in the measurement of the particle’s momentum and (say h-bar) is the h reduced Planck’s constant 2 5 **A consequence of the uncertainty principle is that measuring the state of a microscopic system changes the system.** Consider measurement in the macroscopic world To measure position of a cart at a specific time, we need to measure with our eye or a photograph. - Either instrument uses photons reflected off the cart to image the cart. To measure momentum, we need two photographs separated by time. Consider measurement in the microscopic world. Since photons have momentum, photons reflected off electron will the momentum of the electron. Measuring the momentum using two photographs is impossible. position of particle at time 1 position of particle position of particle at time 1 at time 2 position of particle at time 2 ‘actual’ path of particle apparent path of particle - Using photons, we have no way knowing the actual path between position 1 and position 2. Uncertainty principle also relates uncertainty of time and energy. E t 2 The uncertainty principle is a consequence of wave-particle duality. It cannot be ‘fixed’ with more sophisticated technology. It is fundamental limitation on measurements. Quantum States/Wavefunctions A proper description of a state allows all the known properties of a system to be found. For a macroscopic system, the state is described by a trajectory, r r x, t . - A particle’s momentum, energy, etc… can be found from the trajectory. For a thermodynamic system, the state is described by the equation of state. For a microscopic system, the state is described by a wavefunction, x, t - Unlike trajectory, wavefunction has no independent reality - Wavefunction is with operators to find particle properties, but the value of the wavefunction itself has no physical meaning. Erwin Schrödinger in 1926 developed the idea of a wavefunction. – more very soon. - Nobel - 1933 6 Born’s Interpretation of the Wavefunction has no physical meaning. Only (* ) has meaning as a probability density. - * is complex conjugate of . - * 2 Probability density has physical meaning when associated with a region in space. - infinitesimal regions such as dx, dA, d, etc… probability } dx x2 Probability that electron is between x1 and x2 is prob. * dx x1 The probability that the electron is somewhere must be one. Thus prob. * dx 1 - This requirement implies that the wavefunction must be normalized. Operators and Expectation Values Information is extracted from a wavefunction with an operator. Every observable (measurable quantity) has an operator. Two fundamental operators Position x̂ x Momentum p̂ x i x - caret, ^, is used to denote operator. The information available in a wavefunction is extracted by using the operator to calculate an expectation value. The expectation value of an operator, Ô , is defined as Oˆ * Oˆ d * d If the wavefunction is normalized, i.e., prob. * dx 1 , then the expectation value is Oˆ * Oˆ d - note that the operator must be “sandwiched” between the wavefunctions. 7 Eigenfunctions and operators Often quantum mechanical states can be described with eigenfunctions of an operator. If a wavefunction is an eigenfunction of an operator, then to extract information from the wavefunction with the operator, one needs only to apply the operator upon the wavefunction. Example: We will soon see that the wavefunction of a particle with no potential energy (i. e., a particle in free space) is eikx . This wavefunction is an eigenfunction for the momentum operator. To find the eigenvalue of the momentum operator (i.e., to calculate the momentum) of a particle in free space, one needs to apply the momentum operator to the wavefunction. eikx ˆ ikx pˆ pe d ikx e ik eikx k eikx k p i dx i Commutators and commuting variables Return to Heisenberg’s uncertainty principle x p 2 Since we cannot know position and momentum precisely at the same time, we would expect the expectation value of the position times momentum to be unclear. ˆ ˆ d ?? xp * xp Similarly, ˆ ˆ d ?? px * px ˆ ˆ 0 . The ˆ is different than px ˆ ˆ . Thus xp ˆ ˆ px Later we will show that x̂p ˆ ˆ is known as ˆ ˆ px position and momentum operators do not commute. xp the commutator of the position and momentum operators. Commutators may be form between any two variables. Commutator notation ˆ ˆ x, ˆ ˆ px ˆ pˆ xp Let us now calculate the commutator of x̂ and p̂ . ˆ ˆ xp ˆ ˆ x ˆ pˆ xp ˆ ˆ px ˆ ˆ px x x, i x i x x x x x i i x i x i x i i x i 8 A Theorem of Quantum Mechanics ˆ ˆ BA ˆ ˆ 0 ), then the If and only if operators  and B̂ commute (i.e. AB values of the observables A and B can be known precisely, simultaneously. Therefore, note that this theorem is consistent with Heisenberg’s uncertainty principle. Introduction to the Schrödinger equation. The Schrödinger equation is the fundamental equation of quantum chemistry. The equation has the form of an eigenvalue/eigenvector problem. Classically, the total energy of a system is written in a system’s Hamiltonian, H. A Hamiltonian is a classical quantity that is defined as H = T + V. T – kinetic energy V – potential energy Kinetic Energy Operator 1 T mv 2 2 p p mv v m 2 1 p mp 2 p 2 T m 2 m 2m 2 2m Thus, the Hamiltonian can be written as H p2 V 2m Schrödinger in 1926 replaced p with p̂ and used the Hamiltonian as an energy operator. In one dimension, the kinetic energy operator is 2 2 p2 1 2 T 2m 2m i x 2m x x 2m x 2 2 2 In three dimensions, the kinetic energy operator is 2 2 2 2 T 2m x 2 y2 z 2 The del squared operator, 2 , is a shorthand notation for the second derivative. In Cartesian coordinates, the del squared operator is 2 2 2 2 2 2 2 x y z Note: In other coordinate systems, 2 is much more complicated. Overall, the quantum mechanical energy operator (Hamiltonian) can be written as Hˆ 2 2m 2 V When the Hamiltonian is applied to an eigenfunction of the Hamiltonian, the energy eigenvalue results. 9 Thus, the Schrödinger equation can be written as Hˆ E Requirements of the Wavefunction 1.) Must be normalizable (square-integrable) N * N d 1 N 1 * d 2.) 3.) 4.) 5.) 6.) Must be continuous Must have continuous first derivatives Must be finite First derivative must be finite. Must be single valued - wavefunction must be a function of x and t. 7.) First derivative must be single valued. Note the requirements on the first derivative are necessary to make the kinetic energy operator a physically meaningful operator. Hilbert Space x x x x x Recall matrix eigenvalue problem. x x x x x x x x x x 3 3 matrix yielded 3 different eigenvectors As an unproven assertion, let us state that any point in 3-space can be described by the addition of the appropriate number of eigenvectors. - 3-dimensional space is “spanned” by the eigenvectors For the Schrödinger equation, H E , all possible wavefunctions, , can be found. The set of wavefunctions, , spans the multi-dimensional Hilbert space - The Hilbert space is often has infinite dimension, as is the case for the Schrödinger equation eigenfunctions. 10 Basis Sets Consider three-dimensional space. Any vector in 3-space can be described as a combination of the unit vectors, ˆi, ˆj and kˆ The vectors, ˆi, ˆj and kˆ , form a basis set for three-dimensional space. Now let us extend the idea to Hilbert space. The energy eigenfunctions of the Schrödinger equation are the “unit vectors” of Hilbert space. Thus, any arbitrary wavefunction within Hilbert space can be described as a combination of the energy eigenfunctions. Orthonormality of Wavefunctions The basis set in three-dimensional space is orthogonal to each other, that is, ˆi ˆj 0 ˆj kˆ 0 kˆ ˆi 0 The basis set is also normalized ˆi ˆi 1 ˆj ˆj 1 kˆ kˆ 1 Because the unit vectors are orthogonal and normalized, the unit vectors are orthonormal. The orthogonality and normalization properties can be defined for the basis sets in Hilbert space. a and b are defined to be orthogonal to each other in Hilbert space if and only if, d 0 d * a b * b a Most often, a basis set for a particular operator is constructed so that the wavefunctions are orthonormal. d * a b ab 0 if a b where ab 1if a b - ab is known as Kronecker’s delta. One example of an orthonormal basis set with which we have some experience is that of the orbitals of the hydrogen atom. We will consider many different basis sets. Each basis set will depend on the specific operator being considered.








