Class Question
advertisement
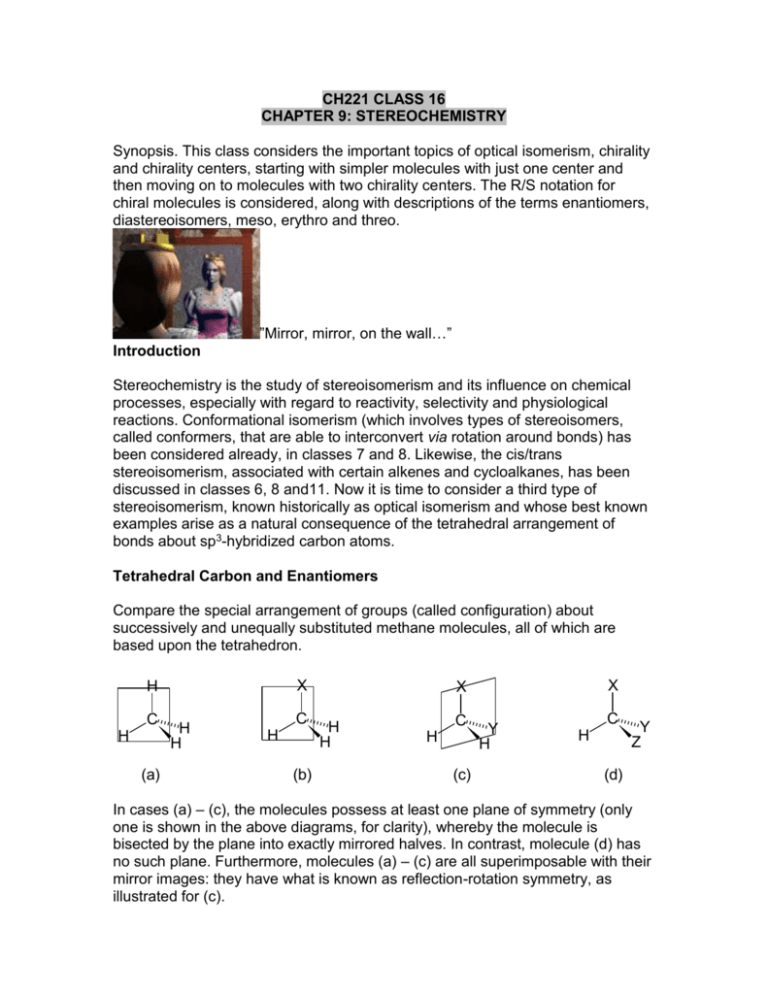
CH221 CLASS 16 CHAPTER 9: STEREOCHEMISTRY Synopsis. This class considers the important topics of optical isomerism, chirality and chirality centers, starting with simpler molecules with just one center and then moving on to molecules with two chirality centers. The R/S notation for chiral molecules is considered, along with descriptions of the terms enantiomers, diastereoisomers, meso, erythro and threo. ”Mirror, mirror, on the wall…” Introduction Stereochemistry is the study of stereoisomerism and its influence on chemical processes, especially with regard to reactivity, selectivity and physiological reactions. Conformational isomerism (which involves types of stereoisomers, called conformers, that are able to interconvert via rotation around bonds) has been considered already, in classes 7 and 8. Likewise, the cis/trans stereoisomerism, associated with certain alkenes and cycloalkanes, has been discussed in classes 6, 8 and11. Now it is time to consider a third type of stereoisomerism, known historically as optical isomerism and whose best known examples arise as a natural consequence of the tetrahedral arrangement of bonds about sp3-hybridized carbon atoms. Tetrahedral Carbon and Enantiomers Compare the special arrangement of groups (called configuration) about successively and unequally substituted methane molecules, all of which are based upon the tetrahedron. X H C H (a) H H C H (b) X X H H C H (c) Y H C H Y Z (d) In cases (a) – (c), the molecules possess at least one plane of symmetry (only one is shown in the above diagrams, for clarity), whereby the molecule is bisected by the plane into exactly mirrored halves. In contrast, molecule (d) has no such plane. Furthermore, molecules (a) – (c) are all superimposable with their mirror images: they have what is known as reflection-rotation symmetry, as illustrated for (c). X reflect in mirror C H Y X Y H H C H (c) identical mirror plane X C H Y H rotate (c) Molecule (d), however, is not superimposable with its mirror image, that is to say it lacks reflection-rotation symmetry. In the diagram below, (d’) is not the same as (d) and hence molecules like (d) can exist in two stereoisomeric forms whose configurations are non-superimposable mirror images. reflect in mirror X C H Y Z X Y Z C H (d) X not identical C H Z Y rotate (d') In general, mirror image molecules that are not superimposable are called enantiomers. Alternatively, we can say that enantiomers are stereoisomers whose configurations are (non-superimposable) mirror images. Chirality - Handedness in Molecules Molecules that are not superimposable on their mirror images and hence exist in two enantiomeric forms are said to be chiral (from Greek cheir, meaning hand). Molecules that do not have the above property are called achiral. The most common cause of chirality in organic chemistry is the presence of an sp3-hybridized carbon atom that is bonded to four different groups. Such carbons are called chirality centers, but many alternative names have been used, such as asymmetric carbon atoms, asymmetric centers and stereogenic centers. Some examples are given below. no plane of symmetry CH3 H C CH3 H Propanoic acid H COOH C OH 2-Hydroxypropanoic acid (lactic acid) COOH plane of symmetry Exists as two enantiomeric forms: Exists in only one (achiral) form HO OH C CH3 COOH H CH3 H CH3 H Methylcyclohexane (achiral) HOOC H C CH3 O 2-Methylcyclohexanone (chiral) Some organic chiral molecules, not discussed here, do not actually have a chirality center, but all chiral molecules lack reflection-rotation symmetry, or more simply, lack a plane or center of symmetry. However, in determining whether or not a molecule is chiral, it is useful initially to look for the presence of chirality centers. Quite often, it will be necessary to look a long way from the chirality center (which can be indicated with an asterix, *), as illustrated by the example below. Br CH3CH2CH2CH2CH2 C* CH2CH2CH2CH3 H 5-bromodecane (chiral) Class Question Identify the chirality centers in the following molecules. Use *. Optical Activity Jean Baptiste Biot was the first to discover that many (but not all) natural substances have the ability to rotate the plane of plane-polarized light as it passes through. The angle through which the plane is rotated is called the angle of rotation, , and is measured using an instrument known as a polarimeter: Substances that rotate the plane of plane-polarized light to the right (clockwise) are called dextrorotatory (d) and are given the symbol (+). Those rotate the plane to the left (anticlockwise) are called levorotatory (l) and are given the symbol (-). More useful than optical rotation is specific rotation, whose symbol is []tD, where D is the wavelength of the sodium D-line (589 nm) that is commonly used in polarimeter experiments and t is the temperature in o C. Specific rotation is defined below. See Table 9.1 on p. 281 of the textbook for examples of the specific rotations of common organic substances. Louis Pasteur The great chemist and pioneer biochemist/microbiologist Louis Pasteur was first to relate rotation of plane-polarized light to molecular structure. Pasteur was able to isolate mirror image crystals of sodium ammonium tartrate from a concentrated solution by crystallization below 28 oC. He showed, using magnification and tweezers, that the right-handed crystals of sodium ammonium tartrate, when dissolved in water, rotated the plane in one direction, whereas a solution of equal concentration made from the left-handed crystals rotated the plane equally in the opposite direction. Through Pasteur’s work, the phenomenon described above became known as optical isomerism, but it was not until 25 years later that Joseph Le Bel and Jacobus van’t Hoff demonstrated that chiral molecules were the only molecules capable of showing this phenomenon – i.e. only chiral molecules are capable of optical activity. R/S Notation for Chiral Molecules Following the work of Le Bel and van’t Hoff, the need quickly arose for a chiral nomenclature – a naming system that unambiguously tells us the configurations of chiral molecules. At the end of the 19th century, Emil Fischer proposed the D/L notation. By assuming that D-(+)-glyceraldehyde has the following configuration, CHO C HOCH2 OH H , he and others were able to notate the configuration with the sign of rotation of a large number of molecules, but it was not until 1951 that Bijvoet, using X-ray crystallography, proved (luckily) that Fischer was correct. However the D/L notation has been partly superceded by the less ambiguous R/S notation, based upon the Cahn-Ingold-Prelog sequence rules that are also used for the Z/E notation for ‘geometric isomerism’. To name a chiral configuration according to the R/S notation, carry out the following steps. 1. Assign priority labels (1, 2, 3, 4 or a, b, c, d) to each of the four groups attached to the chirality center, according to the Cahn-Ingold-Prelog rules described for E/Z notation. E.g. (+)-lactic acid 2. View the molecule from the side remote from the lowest priority group (number 4, here, H). E.g. 3. Name R (Latin rectus, for right-handed) if the sequence (1) (2) (3) is clockwise (right-handed) or S (Latin sinister, for left-handed) if anti-clockwise (left-handed). 1 2 E.g. in the above example, C anticlockwise 3 , The sequence is anticlockwise and hence the configuration of (+)-lactic acid is S: its full name is (S)-(+)-lactic acid. Class Question. Determine the configurations of the following compounds, according to the R/S notation. CHO H (a) (+)-Glyceraldehyde (b) (-)-alanine C C CH2OH COOH H H2N OH CH 3 Stereoisomerism of Molecules Possessing Two Chirality Centers So far, we have considered molecules with only one chirality center – now it is time to study molecules with two chirality centers. Two specific cases will be dealt with here: firstly, when the two sets of groups attached to the chirality centers are different and secondly, when there are identical sets of groups attached to the two chirality centers. The important stereochemical terms enantiomers, diastereoisomers, internal symmetry, meso, erythro and threo are introduced and defined, although the first term has already been mentioned earlier in the course. Two Chirality Centers Possessing Non-identical Sets of Groups Consider the ALDOTETROSE sugars (2,3,4-trihydroxybutanal, OHC.CH(OH).CH(OH).CH2OH). The FOUR POSSIBLE stereoisomers are represented below. CHO CHO H C H C R R OH HO C OH HO C S S CHO H H C H HO C R S CHO OH H HO C H C S R H OH CH2OH CH2OH CH2OH CH2OH 1 2 3 4 OH OH H H HO HO CHO CH2OH H HO H H OH H HO H CHO CH2OH CHO CH2OH H OH CHO CH2OH Newman Formulas There are no internal mirror planes and so each structure represents an individual stereoisomer. In fact, 1 and 2 are (-)- and (+)-erythrose, respectively; 3 and 4 are (+)- and (-)-threose, respectively. ERYTHRO and THREO are terms used for configurations as follows: a C b a C b a C b b C a Erythro Threo A look at the four structures above reveals that there are two kinds of isomers here. Firstly, there are those whose configurations are related by MIRROR REFLECTION: these are 1 and 2, and also 3 and 4. They are called ENANTIOMERS. Secondly, there are isomers whose configurations are not related by mirror reflection: these 1 and 3, 2 and 3, 1 and 4, and 2 and 4. They are called DIASTEREOISOMERS. Two Chirality Centers with Identical Sets of Groups Consider TARTARIC ACID (2,3-dihydroxybutanedioic acid, HOOC.CH(OH).CH(OH).COOH). The FOUR POSSIBLE stereoisomers are represented below. Newman Formulas It can be seen that isomers 1 and 2 are IDENTICAL - they are superimposible. This is because both structures possess INTERNAL SYMMETRY (an internal mirror plane (imp), .......). Both 1 and 2 represent the achiral MESO-TARTARIC ACID, which is not found in nature. Isomers 3 and 4 are CHIRAL (they are enantiomeric) and represent (+)- and (-)-TARTARIC ACID, respectively. Thus, an important general situation exists for molecules of the type xyzC-Cxyz – only three stereoisomers exist (out of a possible four), one meso isomer which is optically inactive and a pair of enantiomers. Class Question Does cis-1,2-dimethylcyclobutane have any chirality centers? Is it chiral? cis-1,2-Dimethylcyclobutane CH3 CH3 H H Yes, the molecule has two chirality centers (*), but is achiral, because it possesses a plane of symmetry ( ): * CH3 H * CH3 H