COLLIGATIVE PROPERTIES - FREEZING POINT DEPRESSION
advertisement

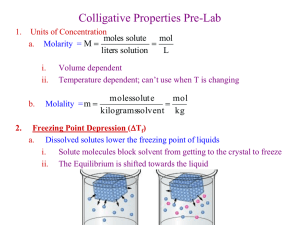
1 COLLIGATIVE PROPERTIES - FREEZING POINT DEPRESSION INTRODUCTION As would be expected, when a solute is dissolved in a solvent, the resulting properties of the solution are very different than those of the pure solvent. Solutions exhibit a lower vapor pressure, a higher boiling point, and a lower freezing (melting) point than the pure solvent and also exhibit osmotic pressure. These properties are called colligative properties and their magnitude depends entirely on the concentration of moles of solute particles and not on the chemical identity of the solute particles. When dissolved in a given solvent and in a given quantity of the solvent, a mole of sodium ions, a mole of sulfate ions, a mole of sugar molecules and a mole of albumin (a protein) molecules, would each lower the vapor pressure, raise the boiling point, or lower the freezing (melting) point to the same magnitude and each solution would also have the same osmotic pressure. This phenomenon is closer to ideal in dilute solutions rather than concentrated ones. Colligative properties are useful in counting moles of a solute, even when the identity of the solute is not known. If one can count the number of moles of a substance in a given number of grams of that substance, the molar mass is easily calculable. The magnitude of the lowering of the freezing point is directly related to the concentration of the solution and can be expressed mathematically by the expression, ΔTf = -kf m, where ΔTf represents the change in the freezing point (Tf of the solution minus the Tf of the pure solvent). Since the freezing point of the solution is always lower than that of the pure solvent, Tf is always negative and thus the minus sign. Kf is a constant for any given solvent and is called the molal freezing point depression constant. The value for kf for water is 1.86 o -1 C or oC kg solvent/mol of solute. The m represents the molality of the dissolved solute particles in the solution and is the moles of solute particles per kilogram of solvent. Molality and mole fraction are used for vapor pressure, boiling point and freezing point studies because they are independent of temperature as opposed to molarity, which is temperature dependent. The greater the concentration of the solution, the lower the freezing point will be. In solutions made with solutes that dissociate when dissolved in water, the molality of dissolved particles is always greater than the molality of the solution. In other words, NaCl forms Na+ and Cl- in water so a 1 molal NaCl solution would behave as a 2.0 molal solution. Sugar molecules dissolve without dissociation, so a 1 molal sugar solution behaves like a 1 molal solution. In fact this methodology is useful for determining the degree of dissociation for weak electrolytes. Anything dissolved in water serves as an antifreeze and the more antifreeze the lower the freezing point. When salt is put onto sidewalk ice, it dissolves to form a very concentrated solution. If the surrounding ice has a temperature higher than the freezing point of the salt water, it will melt. Melting, of course, requires energy and the energy comes from the surroundings making them colder. In this exercise, the freezing points of a variety of aqueous solutions will be measured and the molar mass of glycerin will be determined using freezing point depression data. SAFETY CONSIDERATIONS None of the materials used in this exercise are hazardous. DISPOSAL OF MATERIALS All materials can be flushed down the drain with water. f. p. depression 2 Name____________________date_______ PROCEDURE I. Preparing an ice bath 1. Place about 300 mL of crushed ice in a 600 beaker. 2. Add about 6 grams (heaping teaspoon) of NaCl to the ice and stir the mixture vigorously and record the temperature to the nearest 0.1 degree. 3. Add three more successive 6 g samples of NaCl to the mixture, stirring and recording the temperature after each addition. II. Freezing point of pure water (calibration of the thermometer) 1. Place about 10 mL of distilled water into a clean large test tube, equipped with a thermometer and copper wire stirrer which fits around the thermometer.. 2. Chill the water in the test tube with the ice bath until there is some ice in the tube. Stir the water in the tube vigorously. DO NOT FREEZE IT SOLID. Record the temperature of a liquid - solid equilibrium as the freezing point of the pure water. III. Freezing points versus concentration of NaCl 1. To 10.0 mL (0.010 kg) of distilled water in a clean test tube, add 0.59 grams(0.01mol) of NaCl. 2. Measure and record the freezing point of the solution by chilling with vigorous stirring in the ice bath. The more water that freezes in the mixture, the more concentrated the solution becomes and the lower the freezing point becomes. Therefore, the desired freezing point is determined when the first crystal of ice forms and the concentration of the solution is known. This is very difficult to determine. It is best done by chilling the solution with vigorous stirring until there are some ice crystals in the tube and then removing the tube from the ice bath, allowing the solution to warm while stirring vigorously and reading the temperature when the last crystal of ice melts. The tube can be lowered into the ice bath and the process repeated. Several reading can thus be averaged. Some practice is usually required to obtain good freezing point data. 3. Add another 0.59 g sample of NaCl to the 1.0 m NaCl solution and repeat the above freezing point measurement. Record the data. 4. Repeat step 3 making the solution 3 molal. IV. Freezing point of a sugar solution. 1. Determine and record the freezing point of a 2.0 molal (342 g/mol) sugar solution using the above methodology. To 10.0 mL (0.010 kg) of distilled water in a clean test tube, add 6.84 grams(0.02mol) of sugar. V. Molar mass of glycerin. 1. Accurately weigh out a 2.50 to 3.0 gram sample of glycerin into a clean large test tube. BE CAREFUL NOT TO SPILL GLYCERIN ON THE BALANCE. 2. Add 10.0 mL of distilled water to the glycerin and dissolve it. 3. Determine and record the freezing point using the above methodology. DATA, CALCULATIONS AND ANALYSIS I. Preparing the ice bath Temperature with 6 g NaCl. _____ 12 g _____ 18 g _____ 24 g _____ Why is there a minimum temperature that can be achieved with ice in salt water? 3 What is the minimum temperature in degrees Celsius? ________ in degrees Fahrenheit? _________ 4 f.p.depression Name____________________date_______ II. Freezing point of pure water (calibration of individual thermometers) What is the freezing point of pure water? ________ What temperature does the thermometer read at this temperature (nearest 0.1 degree)? ________ III. Freezing point versus concentration of NaCl 0.5 m Reading # 1 ______ # 2 ______ Average _______ ΔTf ______ 1.0 m Reading # 1 ______ # 2 ______ Average _______ ΔTf ______ 2.0 m Reading # 1 ______ # 2 ______ Average _______ ΔTf ______ What does this data indicate regarding the dissociation of NaCl in water? IV. Freezing point of a sugar solution 1.0 m Reading # 1 ______ # 2 ______ Average _______ ΔTf ______ How does this data compare to the ΔTf of the 1.0 m NaCl solution What does this data indicate regarding the dissociation of sugar in water? V. Molar mass of glycerin Mass of glycerin __________ Freezing point of solution. Reading # 1 ______ # 2 ______ Average _______ ΔTf ______ Molality of glycerin solution using Tf= Kf m _________ Molar mass of glycerin using your experimental data __________ The formula for glycerin is C3H8O3. What is the % error in your determination? ________ QUESTIONS 1. Why does ice water get colder when salt is added and how does salt help melt sidewalk ice if it lowers the temperature? 2. To what minimum temperature would a radiator be protected if equal volumes of ethylene glycol (permanent antifreeze) (density 1.11 g/mL) and water were mixed. Assume that ethylene glycol does not dissociate and has a formula, C2H6O2. 3. A 7.85 g sample of a compound with an empirical formula C1H1 is dissolved in 172 g of benzene. The freezing point of the solution is 1.50 C below that of pure benzene. Assuming that the compound does not dissociate, what is the molar mass and molecular formula of this compound? The freezing point depression constant for benzene is 5.12 C/m. _____________________g/mole C_____H_____