Physics 3U Nuclear Decay
advertisement

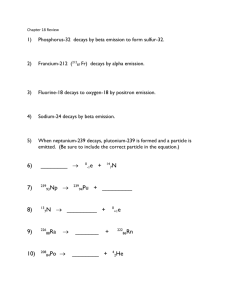
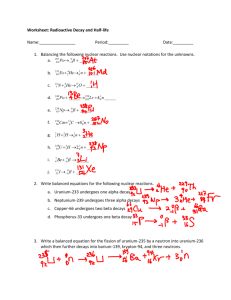
Physics 3U Nuclear Decay/Half-life questions 1. Uranium – 238 decays by alpha particle emission. What element (name and mass number) is created? 2. Americium – 241 decays by alpha decay. What new element is created? 3. Carbon – 14 decays by beta decay. What new element is created? 4. Cesium – 137 decays by beta decay. What new element is created? 5. Postulate a decay that would turn another element into gold. 6. Carbon – 14 is used for radioactive dating. The half-life of carbon – 14 is 5730 years. If 3g of C-14 was in a sample 2000 years ago, how much C – 14 is in the sample now? 7. The half-life of sodium – 25 is 59.1 seconds. How long would it take for 16g of sodium – 25 to be reduced to 1g of sodium – 25? 8. Sodium – 25 decays by beta decay. What new element is created? 9. The half-life of uranium – 232 is 68.9 years. How much of a 1 kg sample would be left after 100 years? Answers: 1. thorium – 234 2. neptunium – 237 3. Nitrogen – 14 4. barium – 137 5. beta decay of platinum, alpha decay of thallium 6. 2.35g 7. 236 seconds 8. magnesium – 25 9. 365g