Quantum chemical calculations
advertisement
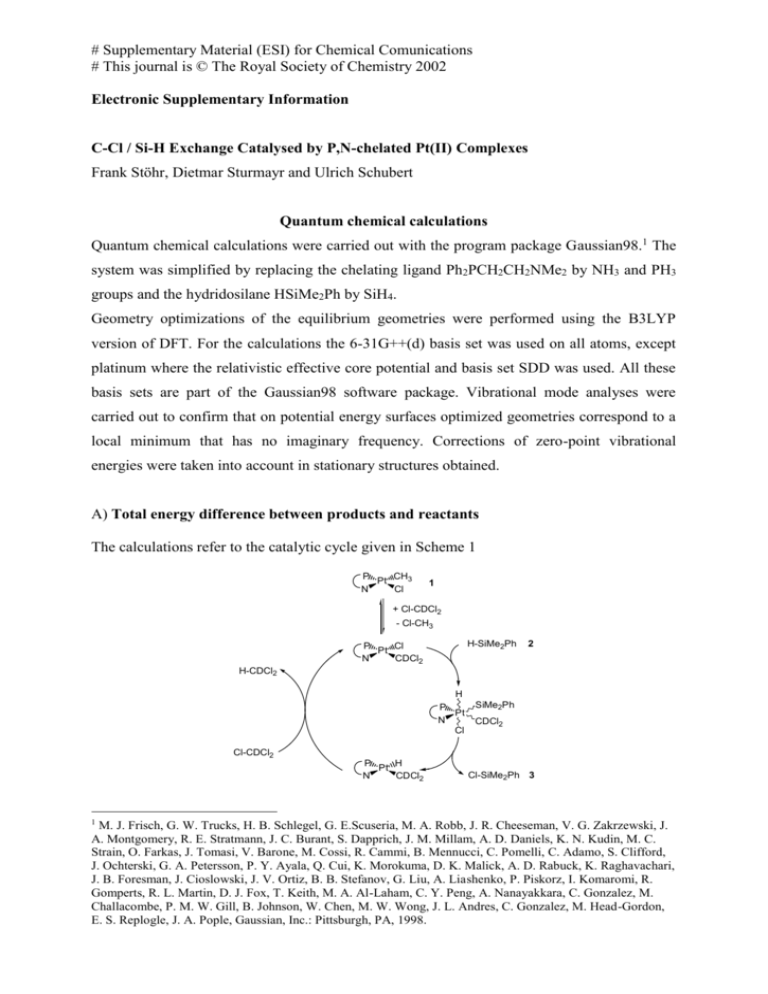
# Supplementary Material (ESI) for Chemical Comunications # This journal is © The Royal Society of Chemistry 2002 Electronic Supplementary Information C-Cl / Si-H Exchange Catalysed by P,N-chelated Pt(II) Complexes Frank Stöhr, Dietmar Sturmayr and Ulrich Schubert Quantum chemical calculations Quantum chemical calculations were carried out with the program package Gaussian98.1 The system was simplified by replacing the chelating ligand Ph2PCH2CH2NMe2 by NH3 and PH3 groups and the hydridosilane HSiMe2Ph by SiH4. Geometry optimizations of the equilibrium geometries were performed using the B3LYP version of DFT. For the calculations the 6-31G++(d) basis set was used on all atoms, except platinum where the relativistic effective core potential and basis set SDD was used. All these basis sets are part of the Gaussian98 software package. Vibrational mode analyses were carried out to confirm that on potential energy surfaces optimized geometries correspond to a local minimum that has no imaginary frequency. Corrections of zero-point vibrational energies were taken into account in stationary structures obtained. A) Total energy difference between products and reactants The calculations refer to the catalytic cycle given in Scheme 1 P CH3 Pt N Cl 1 + Cl-CDCl2 - Cl-CH3 H-SiMe2Ph P Cl Pt N CDCl2 2 H-CDCl2 H P Pt N Cl SiMe2Ph CDCl2 Cl-CDCl2 P H Pt N CDCl2 1 Cl-SiMe2Ph 3 M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E.Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle, J. A. Pople, Gaussian, Inc.: Pittsburgh, PA, 1998. # Supplementary Material (ESI) for Chemical Comunications # This journal is © The Royal Society of Chemistry 2002 Activation Step Pt H3N Cl PH3 CH3 + Cl-CH3-xClx H3N ClxH3-xC PH3 Cl + Cl-CH3 Pt Relative Energy [kcal/mol] Cl-CH3 +9.0 Cl-CH2Cl Cl-CHCl2 Cl-CCl3 +0.5 -4.7 -1.6 Step 1 H3N ClxH3-xC PH3 Cl Pt + H-SiH3 H3N ClxH3-xC Pt PH3 + Cl-SiH3 H Pt PH3 Cl + H-CHyCl3-y Relative Energy [kcal/mol] H3N H3C Pt PH3 Cl -9.4 H3N ClH2C Pt PH3 Cl -9.5 H3N Cl2HC Pt PH3 Cl -10.1 H3N Cl3C Pt PH3 Cl -14.6 Step 2 H3N ClxH3-xC Pt PH3 H + Cl-CHyCl3-y H3N ClxH3-xC Relative Energy [kcal/mol] Cl-CH3 Cl-CH2Cl Cl-CHCl2 Cl-CCl3 H3N H3C Pt PH3 H -2.5 -7.6 -10.0 -14.4 H3N ClH2C Pt PH3 H -2.3 -7.4 -9.8 -14.2 H3N Cl2HC Pt PH3 H -1.8 -6.9 -9.3 -13.7 H3N Cl3C Pt PH3 H +2.7 -2.3 -4.8 -9.2 # Supplementary Material (ESI) for Chemical Comunications # This journal is © The Royal Society of Chemistry 2002 # Supplementary Material (ESI) for Chemical Comunications # This journal is © The Royal Society of Chemistry 2002 B) Total energy of possible intermediates Cl and PH3 cis Cl and PH3 trans Energy [au] * Energy [au] * H3N H3C Pt PH3 Cl -1019.174956 H3N Cl Pt PH3 CH3 -1019.189358 H3N ClH2C Pt PH3 Cl -1478.786038 H3N Cl Pt PH3 CH2Cl -1478.794463 H3N Cl2HC Pt PH3 Cl -1938.387684 H3N Cl Pt PH3 CHCl2 -1938.395475 H3N Cl3C Pt PH3 Cl -2397.969058 H3N Cl Pt PH3 CCl3 -2397.977224 * 1au = 27.21 eV = 627.51 kcal/mol * 1au = 27.21 eV = 627.51 kcal/mol H and PH3 cis Energy [au] * H3N H3C Pt PH3 H -559.536653 H3N ClH2C Pt PH3 H -1019.148006 H3N Cl2HC Pt PH3 H -1478.750477 H3N Cl3C Pt PH3 H -1938.339075 * 1au = 27.21 eV = 627.51 kcal/mol # Supplementary Material (ESI) for Chemical Comunications # This journal is © The Royal Society of Chemistry 2002 C) Atomic coordinates of the calculated complexes after geometry optimization # Supplementary Material (ESI) for Chemical Comunications # This journal is © The Royal Society of Chemistry 2002 # Supplementary Material (ESI) for Chemical Comunications # This journal is © The Royal Society of Chemistry 2002 # Supplementary Material (ESI) for Chemical Comunications # This journal is © The Royal Society of Chemistry 2002 # Supplementary Material (ESI) for Chemical Comunications # This journal is © The Royal Society of Chemistry 2002 # Supplementary Material (ESI) for Chemical Comunications # This journal is © The Royal Society of Chemistry 2002