Physics 20 Unit #4 Heat “Calculations Quiz”
advertisement

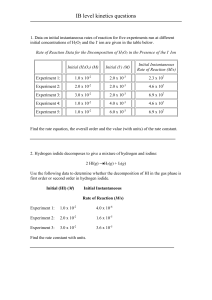
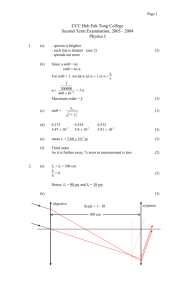
Physics 20 Unit #4 Heat “Calculations Quiz” 1. Brenda heats a 126.9 mm copper rod from a temperature of 275 K to 331 K. Using Table 10-1, calculate how much the rod will change in length. 2. A scientist is experimenting with a new material. He applies a heat change of 36.7 K to an initial length of 15.8 cm. If the material increases by 2.5 mm, what is the coefficient of linear expansion of the material? 3. Frank is testing the change in volume of glycerin under different temperature changes. If he has 23.06 ml of glycerine how much will be in the flask if he changes the temperature from 302 K to 325 K? 4. Tom notices that his mercury changes volume by 3.58 ml when it sits out in the sun. If the initial volume is 4.6 L before expansion, what did the temperature change? 5. A block of Styrofoam has dimensions of 4.2 m x 4.4 m and is 0.13 cm thick. It is used to seal an opening when the inside and outside temperatures are -70 C and 340 C. How much heat escapes through the block in 6 1/4 hours? (See table 10-3) 6. Calculate the temperature change for 1 week of heat loss if 9.34 x 1025 J from a house made of ceramic tile that has an Area of 650m2 and a thickness of 200 cm. (Use table 10-3) 7. Calculate the RSI value for animal fat that is 23.78 cm thick. (Use table 10-3) 8. If the RSI value of silver is 674.13 (m2K)/W. What is the thermal conductivity if the thickness is 25.09 cm? (Use table 10-3) 9. Using Table 11-1, Calculate the amount of the unknown: a) 209.7 g of magnesium warms from 12.560 C to 65.30 C. b)4.76 g of zinc gains 4.34 x 1010 J of heat. c) 34.71 kJ of heat warms 946.02 g of iron from 289 K to 47.60 C. d) 105.88 kJ of heat produces a temperature change of 56.74 K in oxygen. 10. Calculate the heat capacity of the following samples if: a) 77.85 kJ of heat increases the temperature of paraffin oil by 19.4 K. b) 95.26 kg human body. 11. What amount of nickel, at a temperature of 283.50 C, must Tyler add to 7.4 kg of methanol, at a temperature of 155.670 C, to create a mixture at a temperature of 198.10 C (Refer to table 11-1) 12. When Tony adds 450 g of an unknown material at a temperature of 284.8 K to 5.69 kg of ethylene glycol at a temperature of 58.20 C, the final temperature of the mixture is 24.70 C. What is the specific heat capacity of the unknown material? 13. What amount of nitrogen is used if 1.93 x 1013 J of heat is given off during the fusion process? (Refer to Table 11-3) 14. Calculate the amount of heat released when 12.07 g of freon - 14 vaporizes? (Refer to Table 11-3) 15. If 305.81 g of Glauber’s salt gives of 3.93 x 1020 J of energy during the fusion process, determine the latent heat of fusion for the material.