Experiment 2 Mass and Volume Measurements
advertisement

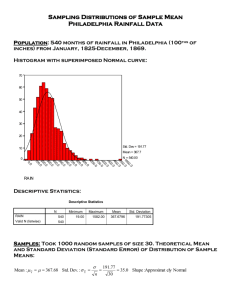
Experiment 2 Mass and Volume Measurements I. Background - Scientific Measurements: Error, precision and accuracy By international agreement reached in 1960, certain basic metric units and units derived from them are to be preferred in scientific use. The preferred units are known as the International System (commonly called SI units from the French, Système Internationale). The basic units of the SI system can be found in Section 1.4 of your text. Another source including historical background is the National Institutes of Standards and Technology (NIST) Internet site (http://physics.nist.org ). Units obtained from the basic units are called derived units. The volume of a cube can be determined by measuring the lengths of its 3 dimensions and computing the volume as the product of those 3 dimensions. Thus volume is derived from length measurements and has the dimensions of length cubed, for example, cubic centimeters (cm3). Experimental Errors Every measurement involves some amount of error (or measurement uncertainty). Because all generalizations or laws of science are based on experimental observations involving quantitative measurements, it is important for a scientist to take into account any limitations in the reliability of the data from which conclusions are drawn. PRECISION AND ACCURACY The error in a measurement is the difference between the true value of the quantity measured and the measured value. Accuracy is a measure of the correctness of a measurement. Unless we have precise standards against which we can test our measurement, we often do not know the true value of a measured quantity. If we do not, we can obtain only the mean, or average value of a number of measurements. A measure of the spread (the range) of the individual values from the mean value is called the deviation ()-defined as the difference between the measured value, xi, and the arithmetic mean, x, of a number, n, of measurements (the symbol xi indicates a particular measurement the ith measurement of quantity x; the bar above a quantity means the average value of the quantity): i = xi – x The smaller the deviations in a series of measurements, the more precise the measurement is. Precision is a measure of the reproducibility of a measurement. qConcept Check: A series of measurements of the length of a piece of wire give x1 = 1.0 cm, x2 = 1.5 cm, and x3 = 1.2 cm. What is the average of the measurements and the deviation of each measurement? Answer: x = 1.2 cm, -0.2 cm 0.3 cm , 0.0 cm In a way similar to Figure 1.25 of your text, we can assess the accuracy and precision of a series of measurements. In Figure 1 below, curve A represents a large number of weighings on less precise balance, and curve B represents a large number of weighings on a more precise balance. The curves are a plot of the number of times a particular measurement occurred vs. that measurement. In this series of measurements, both curves gave the same mean value (2.05 g), so their accuracy is the same. However, the precision of the measurement is much better for balance B; therefore, we can have much more confidence in saying the average mass from balance B is closer to the true value than that of balance A. SYSTEMATIC ERRORS Figure 1. Comparison of the distributions of a number of weighings of a sample on a less precise balance (curve A) and a more precise balance (curve B). There are two general types of errors, systematic and random. A systematic error causes error in the same direction each time a measurement is made. Systematic errors typically affect the accuracy of a measurement, although the precision may remain good. Some examples of causes of systematic error are a miscalibrated scale on a ruler or balance, which would continuously give a high or low reading. If the true value of a reading is unknown, systematic errors can be difficult to detect. RANDOM ERRORS AND STANDARD DEVIATION If a measurement is made a large number of times, you will get a range of values (a distribution) like that shown in Figure 1. The reason is that random errors inherent in any measurement will cause deviations from the average value. For random errors, small errors are more probable than large errors and negative deviations are as likely as positive ones (like that shown in Figure 1). The spread of the data (the degree of imprecision) is expressed by the standard deviation, s (often abbreviated STD): The formula says: Sum the squares of the deviations ( is the mathematical symbol meaning to calculate the sum), divide by n-1 (n is the number of measurements taken), and take the square root of the result. It does sound scary, and indeed, it isn’t much fun to calculate by hand. However, it is a relatively simple process if you follow the 3 steps outlined above. Warning: This formula actually gives an estimate of the standard deviation unless the number of measurements is large (>50). We must recognize that when we repeat a measurement only 2 or 3 times, we are not obtaining a very large sample of measurements, and the confidence we can place in the mean value of a small number of measurements is correspondingly reduced. To estimate the standard deviation for only a few measurements, we can use the average deviation, which is the mean value of the absolute values of the deviation, I: Why bother? The STD or average deviation is a measure of the precision. In Figure 1, curve A had a STD of 0.05 g and curve B had a STD of 0.02 g. You can see that the smaller the STD, the narrower the spread of the data (the more likely will be the probability that any measurement will represent the average value). The narrower the spread of data, the more confidence we have that our data is repeatable. When our data is repeatable, we are more confident that what we measure is a reasonable or true representation of the actual quantity (barring any systematic or other error). What is the difference between accuracy and precision? Why is STD not a measure of the accuracy of a measurement? Why is it necessary to repeat a measurement? That is, why isn’t one measurement of a quantity enough? How many measurements should we take in order to get a minimum degree of confidence in our precision? Things to Think About How Do We Know When Our Precision is Acceptable? Generally, just knowing the STD or average deviation is not enough. We have to realize that if the size of the quantity we are measuring is very large (like the mass of a boulder), then a STD of a few grams is probably not very significant. However, if we were measuring the mass of a small object (like a paper clip) then a STD of a few grams is very significant. But how can we tell? The answer lies is calculating the relative STD (or relative average deviation): You compare the size of your STD with the mean value. If the relative STD comes out to less than 3-5%, your precision is considered acceptable. Expressing Precision: Significant Figures Since we now understand that with any measurement there will be error, we need some way to indicate our precision when we write down our results. This is where we must use significant digits to indicate our precision. Your text and lecture instructor provide ample background on this topic. If you are unsure of how to record sig. figs., consult these sources. Density Ordinary matter, the stuff of which our familiar world is made, has two important properties. We feel its weight when we hold it in our hands, and it takes up space. In the language of science we say that a particular sample of matter has a definite mass and volume. The ratio of these quantities, or mass per unit volume, is called the density, written: In the metric system, this ratio is usually expressed as grams per cubic centimeter (g/cm 3) or its equivalent, grams per milliliter (g/mL). Density is a fundamental property of matter. The measurement of density is necessary for a variety of important procedures in chemistry, as well as serving as an identifying characteristic of any pure substance. An understanding of density is also useful in the everyday world. For example, the charge on your car battery is determined by measuring the density of the sulfuric acid solution in the batter. Professional and amatuer beer brewers and winemakers measure the density of their brew in order to determine the sugar and the alcohol content. (Prost!) Most liquids and solids expand slightly on heating and the volume of a sample of water increases about 4 percent on heating from 4 oC to 100 oC. Water is unusual, however, in that its density increases (volume decreases) slightly as it is heated from 0 oC to 4 oC. At 4oC, water has its maximum density of 1.000 g/cm3. Because density changes with temperature, it is necessary to specify the termperature when reporting density ofa liquid or solid. Water is also unusual in the fact that solid water (ice), is actually less dense than water at 0 oC. These facts are extremely important for life on earth, particularly where fresh water lakes freeze in winter. First, as the surface water of a lake cools, its density increases until it reaches 4 oC, at which point the surface water is at its densest, and sinks to the bottom of the lake. This process brings up warmer water to the surface. Eventually, the lake will all reach uniform temperature, after which the surface water cools down to 0 oC and freezes. Now since the newly formed ice is less dense than the water it froze from, it stays on the surface. Ice is a reasonably good heat insulator, and acts to prevent the water below it from freezing. In fact, the water near the bottom of the lake is often the warmest (near 4 o C). If the ice were denser than the liquid water (as is the case with other pure substances), the ice would sink to the bottom, and new ice would form on the surface, sink, and so on. Eventually, the entire lake would freeze over, ice building from the bottom up. This is bad news if you are a fish or some other aquatic creature. Fortunately, the water in most larger lakes remains liquid even down to air temperatures well below 0 oC (due to the insulation of the ice surface, and the fact that an unusually large amount of heat must be removed to freeze a unit of water, one of the greatest of any substance – water is truly an amazing substance!). This is good news for fish and the crazy ice fisherpeople who drive their trucks on the frozen lakes to fish. Which brings us to the other advantage of knowing about density - its usefulness in brewing beer, which makes ice fishing bearable. BUOYANCY Buoyancy is the tendency of a substance to remain afloat or to rise in a fluid. A cork floats on water. An ice cube also floats, but it does not ride as high out of the water as a cork. Each substance that floats on water finds a level where the mass of the water displaced is equal to the mass of the substance. Based on these observations, which is more dense, ice or a cork? Things to Think About Cork and ice don’t mix with water to form a homogeneous solution (a solution is homogeneous if every part of the solution has the same composition). However, a homogeneous solution can be formed by mixing two solutions that are completely miscible in each other (solutions are said to miscible if they can be mixed together in all proportions to form a homogeneous solution. Water and ethanol (grain alcohol) are two such miscible solutions). What would happen if we added two homogeneous solutions having different densities to one another? If the solutions were completely miscible, would you expect them to immediately mix? Things to Think About We will investigate these questions and others in this laboratory. Purpose To become familiar with the metric units of mass, length, and volume. To measure the densities of liquids and solids. To investigate the relationship between solution density and solution concentration. Procedure I. The Buoyancy and Density of Diet and Regular Coke. (Work in groups) This experiment will be done in groups of 3-4 students. Each student is responsible for recording all data collected by the group. 1. Determine the mass to the nearest 0.1 g of each of six cans of regular Coke. a. Make sure the outsides of the cans are dry. 2. Fill a 2-3 gallon container (or the lab sink on the end of the lab table) with tap water, and place the previously weighed cans in the water. Do the cans sink or float? 3. Repeat steps 1-2 with six cans of diet Coke. Do you observe any differenes in the buoyancy of the cans of regular and diet coke? Things to Think About a. 4. Determine the appoximate density of regular and diet Coke. Determine the average mass and volume of the beverage contained in the can. Sit down with your group and come up with a procedure for determining the mass and volume of the soda in the can. Use any method at your disposal. Clean, dry, empty cans are available to you, as is any regular lab equipment. When you feel you have a procedure, show it to your instructor and have him/her initial below. Include copies of your procedure in your lab report. II. The Buoyancy of Sugar (Sucrose) Solutions (Work in pairs) You will determine if two miscible solutions having different densities can be floated on one another. The two solutions will at first be identified only by their different colors, one red the other green. One solution contains 8.0% sucrose by mass and the other 16% sucrose by mass. (Sucrose is common table sugar). Your job: A. Try and find out which solution is the most dense by trying to float one solution on the other. B. Make accurate density measurements of the two solutions. C. After this, you will be given the composition of the two solutions. You will be asked to see if there is a correlation between the density of a solution and its composition, expressed as mass% sucrose. Equipment (per pair): 2 polyethylene transfer pipets (plastic droppers) 2 large test tubes (from lab drawer) 6 small test tubes (from lab drawer) red and green sucrose solutions (from reagent supply cart) Procedure: 1. Obtain approx. 5 mL of the red and green solutions and place into separate, clean, large test tubes. 2. Put a transfer pipet in each colored solution and suck some of the solution into the pipets. Note: as you fill the pipets, leave the tip below the surface of the solution, an take your fingers completely off the bulb, so that you do not draw air into the stem of the pipet. 3. Put about a 1-cm deep layer of red solution into a clean, dry small test tube. 4. Repeat with the green solution in a different clean, dry small test tube. 5. Repeat with distilled water in a third clean, dry small test tube. Now you will observe and record what happens whenyou ad solution of the opposite color (red to green and green to red). Begin by adding red to green in the following way… 6. 7. 8. 9. 10. Put the tip of the pipet with the red solution in it about 1 millimeter below the surface of the green solution. Gently squeeze the bulb to add the red solution one drop at a time. You will be able to see more clearly what's happening if you view the solution against a white piece of paper. Observe: Do the red drops sink or rise as they are added? Keep adding solution drop by drop until you have added a volume of the red solution that is about equal to the volume of the green solution already in the tube. Repeat the above process, adding green solution to the red solution. View both tubes against a white background. •In which tube is one layer most clearly floating on top of the other? •What conclusions can you draw from these observations? •Which solution has the higher density, the red or green solution? Quantitative Measurement of the Density of Sugar Solutions (Work in pairs) Equipment: 1 10-mL volumetric pipet 6 50-mL Erlenmeyer flasks wax pencil Procedure: •Use the following procedure to determine the density of both the red and green sucrose solutions. •Your instructor will demonstrate the correct way to use a volumetric pipet. •Be sure that you have rinsed out all previous solution before measuring the volume and mass of the next solution. 1. Number each flask 2. Weigh and record the mass of each clean, dry Erlenmeyer flask. How do you clean a volumetric pipet? How do you "prime" a volumetric pipet? Will you blow the last few drops out of the tip? How many decimal places will your data be recorded to? Things to Think About 3. 4. 5. 6. Use the volumetric pipet to deliver 10.00 mL of solution into a previously weighed Erlenmeyer flask. Reweigh the flask plus solution. Measure and record the temperature of the solution. Repeat the measurements with a fresh sample and a fresh flask. How many repeat trials should you do for reliable data? Things to Think About 7. Repeat your set of measurements for the other colored solution. OPTIONAL EXPERIMENT - IF TIME PERMITS IV. The Density of Solid Aluminum (Work in pairs) 1. 2. Obtain an irregularly shaped piece of aluminum metal from the reagent cart. Determine the density of the aluminum metal using a graduated cylinder and balance. Data Analysis I. The Buoyancy and Density of Diet and Regular Coke. 1. 2. 3. 4. 5. Calculate the average mass for each sample of six cans. Is there a significant difference in the average mass of a can of regular and diet Coke? Determine the average deviation, and the relative average deviation for each sample you made multiple measurements of. Comment on whether your precision is acceptable or not. Calculate the density of regular and diet Coke. Compare this density to that of water at the same temperature. Your instructor will have a density table for water, or you can look it up in the CRC Handbook of Chemistry and Physics (the “bible”). Make a statement that describes this comparison. Calculate the mass percentage of sugar in the regular (sugared) Coke, using the manufacturer’s specified sugar content (g sugar) and the volume, and the density you calculated in step 2. Recall that: mass Coke (g) = density (g/mL) x volume (mL). Also: II. The Buoyancy of Sugar (Sucrose) Solutions 1. What conclusion do you draw about which solution is denser, the red or green. Be sure to explain why you drew this conclusion. Quantitative Measurement of the Density of Sugar (Sucrose) Solutions 1. Calculate the density of each of the sucrose solutions (red and green) in g/mL. 2. Record your values for density on the board at the front of the room. 3. Ask your instructor to tell you which is the 8.0% and 16.0% sucrose solution. 4. Determine if there is a simple relationship between density and mass% sucrose by constructing a graph of density (g/mL) vs. mass% sucrose (y vs. x). Ideally, you should use a computer graphing program to make your graph. 5. Include a data point for pure water (0% sucrose). You will have to determine the density of pure water at the temperature of your sucrose solutions by looking it up in a table (CRC or from instructor) 6. Add a best-fit line through the three points. 7. Plot the average values of density using the combined data of all the members of your laboratory section vs. mass% sucrose. (The average of several values is statistically a more reliable measure of the true value of a quantity than any single measurement.) The graphs you made can be used to estimate the sugar content in regular or diet Coke or any common beverage such as apple juice or other fruit juice drinks. (In these drinks, the other main ingredient besides water is sugar). You will use the graph to estimate the sugar content in regular Coke from Part I. 8. On the graph of density vs. mass% sucrose, draw a horizontal line corresponding to the density of regular cola calculated in Part I. Where this line intersects the best-fit straight line, drop a perpendicular line to the mass% sucrose axis and read off the corresponding mass% sucrose. Compare this value with the mass% sucrose you calculated in Part I (g sugar/g Coke x 100). Do the two values agree? What are some possible sources of uncertainty in the measurement of density and mass% sucrose? The Density of Solid Aluminum Determine the density of solid aluminum based on your multiple measurements of mass and volume. Additional Questions (optional) If you add ice at 0 ˚C to a container of water maintained at 0 ˚C, the ice will float on the water with the water finding a certain level in the container. As the ice gradually melts, forming liquid water at 0 ˚C, would you expect the water level in the container to go up, down, or remain the same? Explain the reasoning by which you arrived at your conclusions. The density of diet cola is about the same as pure water. The cola was contained in an aluminum can, and if you did Part IV, you know that the density of aluminum is considerably greater than that of water. Considering all this information and your own experience drinking carbonated beverages, explain how it is that cans of diet cola can float in water while cans of regular (sugared) cola sink? Wouldn't you expect both kinds of cola cans to sink since both diet cola and regular cola have a density equal to or greater than water, and they are contained in an aluminum can that has a density greater than water? Could a battleship float in a bathtub? Suppose you had an enormous bathtub, one that was larger than a battleship (or a very small battleship). In either case, there is just a bit of water all around and under the ship. Specifically, suppose the ship weighs 100 tons (a very small ship) and the water surrounding it in the tub weighs 100 pounds. Will it float or touch the bottom? Choose one of the answers below, then explain your choice! It will float if there is enough water to go all around it. It will touch bottom because the ship's weight exceeds the water's weight.