Vibrational spectroscopy
advertisement
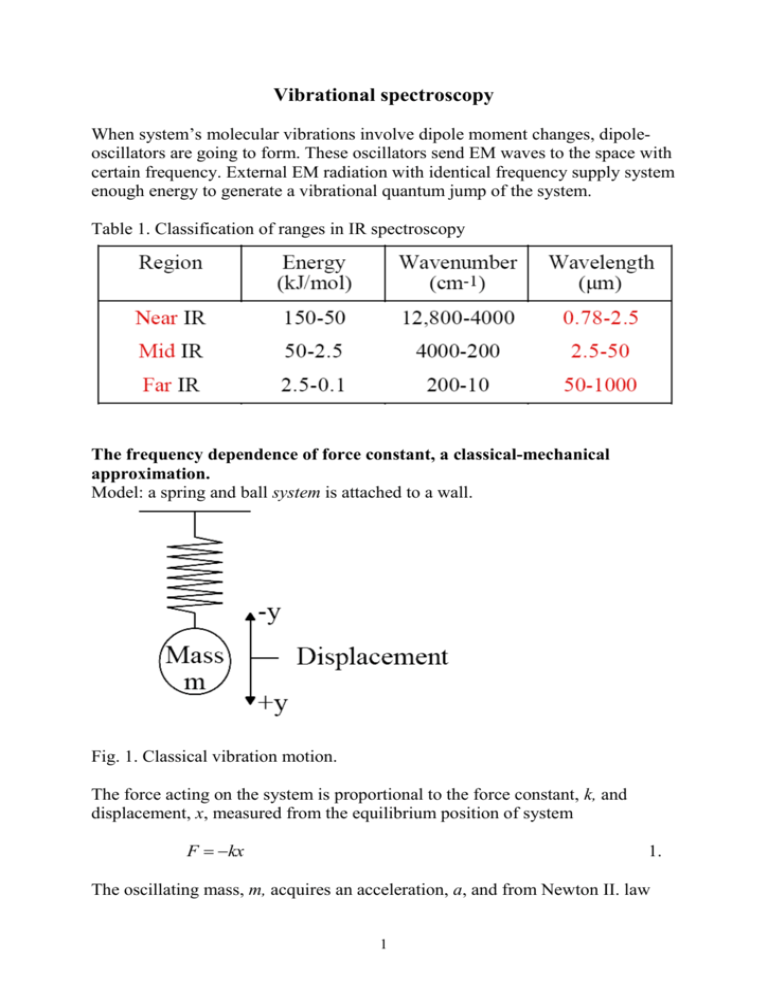
Vibrational spectroscopy When system’s molecular vibrations involve dipole moment changes, dipoleoscillators are going to form. These oscillators send EM waves to the space with certain frequency. External EM radiation with identical frequency supply system enough energy to generate a vibrational quantum jump of the system. Table 1. Classification of ranges in IR spectroscopy The frequency dependence of force constant, a classical-mechanical approximation. Model: a spring and ball system is attached to a wall. Fig. 1. Classical vibration motion. The force acting on the system is proportional to the force constant, k, and displacement, x, measured from the equilibrium position of system F kx 1. The oscillating mass, m, acquires an acceleration, a, and from Newton II. law 1 F ma m d2 x dt 2 2. The solution of this derivative should meet the conditions 1. the function is periodic, because spring and ball system’s movement is periodic. 2. the second derivative of x is equal to the original function multiplied by -k/m. d2 x The second condition: m 2 kx , and dt d2 x k . x m dt 2 3. The function fulfils the first condition if: x A cos2 t b 4. where ν is the frequency of vibration, A is the amplitude of periodic motion and b is the phase of vibration. If b = 0, the first and second derivative of equation 4. dx A2 sin 2 t dt d2 x A42 2 cos2 t 2 dt 5. When Equations 4. and 5. are compared d2 x 42 2 x 2 dt 6. From Equations 3. and 6. we have, x k 42 2 x m 2 and finally we get the frequency dependence: 1 k 2 m 7. The frequency of vibration is linear function of k1/2. The “stronger” the spring the higher its frequency. For molecular system k represents the bond strength. Diatomic molecules Figure. 2. Equilibrium distance: r1, displacement: r2 – r1. For a diatomics with atomic masses m1 and m2 Eq. 7 is modified 1 k 2 1 1 1 m1 m2 7a. where μ is the reduced mass representing a single mass having the same vibrating properties as m1 and m2 produce. 1.1 Harmonic oscillator. When F = -kx law is valid harmonic oscillator approximation can be applied for the description of energetics of molecular vibrations. The displacement, can be defined as (see Fig. 2.), x r2 r1 , and x can be positive, when bond is stretched or that can be negative, when restoring force makes the bond distance less than the equilibrium bond distance. 3 The equilibrium condition: F = 0, x = 0. Potential energy, Vv. dVv F dx 8. F is substituted by -kx dVv Fdx k xdx 1 Vv k x 2 2 or 1 2 Vv k r2 r1 2 9. The graph of this function is a perfect parabola (see Fig. 3.) 1.2 Quantum mechanical energy term According to the law of classical mechanics a diatomics can have any amplitude, consequently can take any potential energy on parabola. By the law of quantum mechanics, the allowed energies of a diatomics are restricted to certain vibrational energies. Ev v 1 / 2 h 10. In equation 10 v represents the quantum number, v = 1, 2, 3 ... . The change in quantum number for a harmonic oscillator is called vibration selection rule: v 1 It means that the vibrating system can exclusively absorb the energy difference between two subsequent vibrational level. The quantum jumps higher than v 2, v 3... are forbidden. The level differences (see also Fig. 3.) E0 0 1 / 2 h E1 1 1 / 2 h E v E1 E0 h 11. 4 The energy difference, E v is quantized, and equal to the energy of exciting photon, h . Equation 11. denotes that the energy difference is independent of the level number, so the levels are equidistant. Vv Harmonic oscillator Anharmonic oscillator 2 1 0 r0 Bond distance Figure 3. Potential energy distance functions, dotted line harmonic oscillator:, solid line anharmonic oscillator. The shape of parabola depends on the force constant (see Eq. 9.). The stronger the bond the higher the force constant, the narrower the parabola. The lengths of a level is equal to twice of the amplitude of the motion. From Eq. 7a the force constant can be given explicitly k 4 2 2 Substituting this result in Eq. 9, we get 1 2 2 Vv k r2 r1 2 2 2 r2 r1 2 12. There is a linear dependence of function Vv f . 1.2 Anharmonic oscillator. The harmonic oscillator approximation refers to the lowest energy vibration levels, where amplitude is small. The anharmonic oscillator approximation models the true function well (solid line on Fig. 3.). A power series gives the energy in wavenumber term. 5 E 2 3 ~ v 0 v 1 / 2 xe v 1 / 2 ye v 1 / 2 hc 12. Where ω0, xe, ye are constants. Selection rule: v 1, 2,3... etc. The quantum jumps v 2 belong to peaks called overtones. Levels are not equidistant, which concludes the form of Eq. 12. At room temperature the 0th energy level is densely populated, therefore the 0 → 1 transition is observed in common, for which harmonic oscillator model fits perfectly well. The intensity of first overtone band is approximately 1/10 of ground vibrational band. Types of Molecular Vibrations The geometry of stretchings: displacements are parallel to the bond. The geometry of bendings: vibrations modify the bond angles.. Stretchings Bendings symmetric asymmetric scissoring wagging rocking twisting/torsion General selection rule: Molecule must have a change in dipole moment due to vibration or rotation to absorb IR radiation. 2.Vibrations of polyatomic molecules, normal modes Introducing normal mode of vibration simplifies the identification of IR bands. For a molecule with N atoms, each atom has three motional degrees of freedom, one each for the translation about the x, y, and z Cartesian axes of the moleculebased coordinate system. Thus, the molecule possesses a total of 3N degrees of freedom. Chemical bonds, which for the moment can be thought of a spring connectors between atoms, serve to constrain the motion of the atoms to well defined vibrational modes. 6 Linear molecules have three unique translations, but only two unique rotations. (The rotation about the bond axis does not count, since it changes neither positions of the atoms, nor does the angular momentum change). Thus, from the total of 3N degrees of freedom, we subtract three translations (on the x, y, and z directions) and two rotations, leaving 3N-5 vibrational degrees of freedom. For a diatomic molecule, 3N-5 vibrational degrees of freedom is consistent with the single vibration along the bond axis (3*2-5=1). Consider now carbon dioxide, O=C=O. The 3N-5 rule for vibrational degrees of freedom predicts 4 vibrations. If the molecule lies along the z-axis, these vibrations are the symmetric stretch, the anti-symmetric stretch, and a symmetric bend in each of the xz and yz planes. It is altogether four. Figure 4. The normal modes for CO2 example. Non-linear polyatomic molecules have three unique rotational degrees of freedom, so they all have 3N-6 vibrational degrees of freedom. Consider now the non-linear molecule water: its three vibrations are the symmetric and antisymmetric O-H stretches, and the bending motion in the molecular plane. All 7 vibrational motions change the molecular dipole moment, so all are infrared active, or electric-dipole allowed transitions. molecule linear non-linear total 3N 3N Mechanical degree of freedom translation rotation vibration 3 2 3N-5 3 3 3N-6 A chemical group can have both stretching and deformational vibration infrared active. In this case the stretching band always appears at greater energies (greater wavenumbers) than the deformational one. E.g. see table below Table Group frequency / cm-1 2970 - 2950 1470 - 1430 1370 - 1365 Functional group / assignment C-H in CH3- / asym. stretch C-H in CH3- / asym. bending C-H in CH3- / sym. bending Fig. 5. Types of molecular vibrations for a triatomic non-linear molecule. 8 3.Isotope effect From Equations 7a and 10 we get E v 1 / 2 h 2 k Turning to wavenumber term 1 k ~ v 1 / 2 2c 13. From assignment of spectral data the wavenumber of an absorption band and the reduced mass of oscillating atoms in the IR range is known. The force constant, k for a particular vibration can be calculated by using Eq. 13. An isotope substitution causes change in reduced mass and consequently in wavenumber of the band position. Suppose, the force constant remains the same by isotope exchange of 35Cl to 37Cl in HCl. The wavenumber shift of H-Cl stretching can be given from Eq. 13. ~ const. μ 1 / 2 Recalling: 1 1 1 μ m1 m2 μ m1m2 m1 m2 For μ given in atomic units: μ 1 H 35Cl μ 1 H 37Cl 1 35 0.9722 1 35 μ H 1 1 37 0.9737 1 37 μ H 1 1 35 Cl 1 37 Cl 1.0142 1.0134 1/ 2 1/ 2 Comparing the results we can say, the lighter isotope (35Cl) has a band at higher wavenumber. 9