Lab Reports - First Four Experiments
advertisement

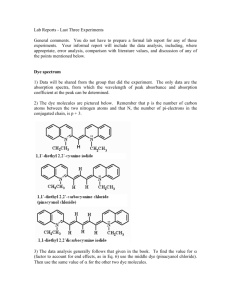
Lab Reports - Last Three Experiments General comments. You do not have to prepare a formal lab report for any of these experiments. Your informal report will include the data analysis, including, where appropriate, error analysis, comparison with literature values, and discussion of any of the points mentioned below. Dye spectrum 1) Data will be shared from the group that did the experiment. The only data are the absorption spectra, from which the wavelength of peak absorbance and absorption coefficient at the peak can be determined. 2) The dye molecules are pictured below. Remember that p is the number of carbon atoms between the two nitrogen atoms and that N, the number of pi-electrons in the conjugated chain, is p + 3. 3) The data analysis generally follows that given in the book. However, I would like you to choose = 0 in your calculations (that is, not use it as an adjustable parameter to improve the agreement between theory and experiment). 4) Make sure to discuss the agreement (or lack of agreement) between the experimental and calculated values for max for the dye molecules. Include a discussion of qualitative agreement (trends in the data) and quantitative agreement (experimental vs model peak wavelength). HCl spectrum 1) Data will be shared from the group that did the experiment. absorption spectra with peak locations. The data are the 2) Begin by making a table of peak assignments and locations for the 1H35Cl and 1H37Cl isotopic species of HCl. Assignments should indicate the value of J in the v"=0 state, and whether it is a P or an R branch transition (so, for example, P(3) or R(5)). Give the peak locations in cm-1 (wavenumbers). 3) Assign values for m to each transition. Recall that m = J" + 1 for the R-branch transitions, and m = - J" for the P branch transitions. You should include the values of m in your table of peak assignments and locations. 4) Data will be fit to Eq. 9 (m) = 0 + (2Be - 2e) m - e m2 where 0, e, and Be are all in units of cm-1. This will mean fitting this equation to a second order polynomial y = a0 + a1 x + a2 x2 x=m y = (m) Also find the 95% confidence limits for your coefficients. 5) Comparison of the above two equations will now allow you to find the values for 0, e, and Be, and the uncertainties in these values at the 95% confidence limits. 6) Compare your experimental values for 0, e, and Be for 1H35Cl with the literature. You may find literature values at the NIST website http://webbook.nist.gov/chemistry/form-ser.html Note that the electronic state is the ground electronic state, labeled X1+ in the NIST table. Also note that you will have to calculate a literature value for 0 using Eq. 8 0 = e - 2 exe 7) Check to see how closely your coefficients for 1H37Cl obey the relationships given in Eq. 11 and Eq. 13. For the comparison in Eq. 13 use 0 instead of e, since you do not have enough information in your experimental results to determine e. Molecular orbital calculations (Handout) 1) Do the molecular orbital calculations for the diatomic molecule for which you did the literature search last semester. That way way, you will already have experimental and theoretical literature values to compare your results with. If you do not have a diatomic molecule ask that one be assigned to you. 2) To get to the screen where you will input the data for the calculation (page 5 of the handout) click FILE---New. 3) Enter the following information % Section Leave blank Route Section #hf/6-31g* opt freq #mp2/6-31g* opt freq #b3yp/6-31g* opt freq Title section You need to put something here or the program will not run So, for example, you could put the kind of calculation (HartreeFock, Moller-Plesset, or Density functional) Hartree-Fock Moller-Plesset Density functional Charge and multiplicity Charge - the charge of your diatomic (will be 0) Multiplicity - 1 (if singlet), 2 (if doublet), 3 (if triplet)... If you don't know this, go back to your literature search results In the section for Molecule Specification enter the data in the same was as in the handout. Li H1R R=1.30 This means the following Li - identifies the first atom in the diatomic H 1 R - identifies the second atom in the diatomic, and tells the program to look for a starting value for R. blank line - R=1.30 - The starting value for R to be used by the program (in Å, angstroms; 1 nm = 10 Å). For your trial value for R you can use the experimental value for re from the literature search, rounded to two significant figures. The program will vary the geometry until it find re, the equilibrium bond distance, so the trial value you put for R doesn't matter (except that if you put something far from the true value for re it will take longer for the program to find the minimum in the potential). 4) After entering the data, click RUN (upper left side of the screen). The program will ask for a file name. I would suggest you use your name and an abbreviation for the type of calculation (joenshf, for example, if I did a Hartree-Fock calculation). 5) It will take about a minute for the program to run, and it will give you a large amount of information. The two things you need are re (equilibrium bond distance) and e (harmonic frequency). Both of these can be found near the bottom of the output file, and will look like the example on pages 7-8 of the handout. 6) Make a table that includes the values for re and e for your three calculations, two calculations taken from the literature, and two experimental values. 7) Briefly discuss your results. Focus on which method seems to best agree with experiment. You should also look at whether your calculations do a better or worse job than the literature calculations in finding re and e.