You will be able to recognize a balanced chemical equation using
advertisement
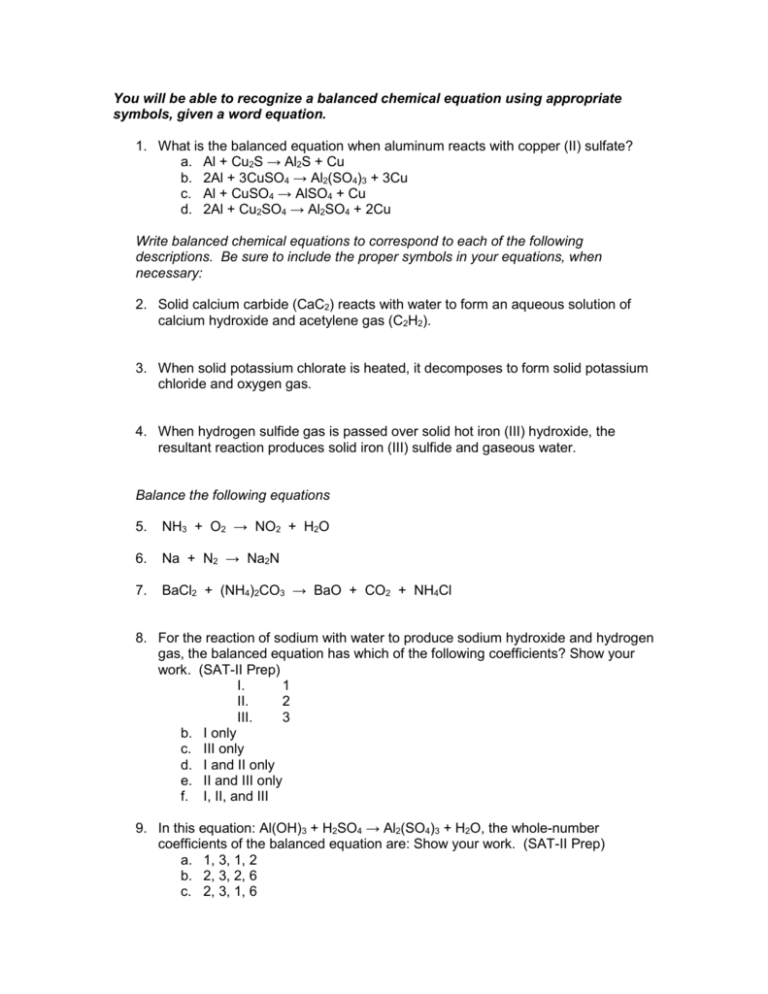
You will be able to recognize a balanced chemical equation using appropriate symbols, given a word equation. 1. What is the balanced equation when aluminum reacts with copper (II) sulfate? a. Al + Cu2S → Al2S + Cu b. 2Al + 3CuSO4 → Al2(SO4)3 + 3Cu c. Al + CuSO4 → AlSO4 + Cu d. 2Al + Cu2SO4 → Al2SO4 + 2Cu Write balanced chemical equations to correspond to each of the following descriptions. Be sure to include the proper symbols in your equations, when necessary: 2. Solid calcium carbide (CaC2) reacts with water to form an aqueous solution of calcium hydroxide and acetylene gas (C2H2). 3. When solid potassium chlorate is heated, it decomposes to form solid potassium chloride and oxygen gas. 4. When hydrogen sulfide gas is passed over solid hot iron (III) hydroxide, the resultant reaction produces solid iron (III) sulfide and gaseous water. Balance the following equations 5. NH3 + O2 → NO2 + H2O 6. Na + N2 → Na2N 7. BaCl2 + (NH4)2CO3 → BaO + CO2 + NH4Cl 8. For the reaction of sodium with water to produce sodium hydroxide and hydrogen gas, the balanced equation has which of the following coefficients? Show your work. (SAT-II Prep) I. 1 II. 2 III. 3 b. I only c. III only d. I and II only e. II and III only f. I, II, and III 9. In this equation: Al(OH)3 + H2SO4 → Al2(SO4)3 + H2O, the whole-number coefficients of the balanced equation are: Show your work. (SAT-II Prep) a. 1, 3, 1, 2 b. 2, 3, 2, 6 c. 2, 3, 1, 6 d. 2, 6, 1, 6 e. 1, 3, 1, 6 10. When the following equation: Cu + HNO3 → Cu(NO3)2 + H2O + NO is balanced, what will be the coefficient, in the lowest number, of Cu? Show your work. (SATII Prep) You will be able to classify a chemical reaction as combination, decomposition, combustion, double replacement, or single replacement and write the appropriate balanced chemical equation. Indicate the letter from the box on your right that best describes the reaction type listed in the following questions. Not all answer choices will be used. 11. ___ Double Replacement 12. ___ Single Replacement 13. ___ Combustion 14. ___ Combination 15. ___ Decomposition a) b) c) d) e) f) X + Y → XY E + XY → EY + X XY → X + Y HY + XOH → H2O + XY X + O2 → YO AB + XY → AY + XB Indicate the type of reaction represented in each of the following unbalanced equations. Answer choices may be used once, more than once, or not at all. 16. ___ ZnCO3 → ZnO + CO2 17. ___ C9H20 + O → CO2 + H2O 18. ___ H2O + N2O5 → HNO3 Li K Ba Ca Na Mg Al Zn Fe Ni Sn H Cu Hg Ag Au 19. ___ KClO3 → KCl + O2 20. ___ LiI + AgNO3 → AgI + LiNO3 g) h) i) j) k) Decomposition Single replacement Double replacement Combustion Combination 21. ___ Ti + 2Cl2 → TiCl4 22. ___ 2C2H2 + 5O2 → 4CO2 + 2H2O 23. ___ H2CO3 → H2O + CO2 24. ___ 3K + FeCl2 → 3KCl + Fe Using the activity series provided on your left when necessary, predict the products of the following: 25. Write a balanced equation given the reactants Li and Cl2. 26. Write a balanced equation given the reactant mercury (II) oxide. 27. Write a balanced equation given the reactants C2H6 and O2 F Cl Br I 28. Will a reaction occur between K and CaO when mixed? If so, write the balanced equation. If no, explain your rationale. 29. Write a balanced equation given the reactants HC2H3O2 and KOH. 30. Cu + CaF2 → 31. Na3PO4 + Ca(ClO2)2 → 32. The reaction of aluminum with dilute H2SO4 can be classified as: Show your work (SAT-II Prep) a. Synthesis b. Decomposition c. Single Replacement d. Double replacement 33. When most fuels burn, the products include carbon dioxide and: (SAT-II Prep) a. Hydrocarbons b. Hydrogen c. Water d. Hydroxide e. Hydrogen Peroxide Explain your answer: __________________________________________ You will be able to determine molar ratios expressed in balanced chemical equations. Given the following balanced equation, answer the questions that follow: 2Al(OH)3 + 3H2SO4 → Al2(SO4)3 + 6H20 34. What is the mole ratio between aluminum hydroxide and hydrogen sulfate (sulfuric acid)? 35. What is the mole ratio between hydrogen sulfate (sulfuric acid) and water? 36. What is the mole ration between water and aluminum sulfate? 37. The unbalanced equation for a reaction is Na2O + H2O → NaOH. Write two mole ratios comparing moles of NaOH and moles of Na2O. a. ___________________________________ b. ___________________________________ 38. Given the following balanced equation, write three possible mole ratios between reactants and products: CaCO3 + 2HCl → CaCl2 + H2O + CO2 a. ___________________________________ b. ___________________________________ c. ___________________________________ Every question below contains two statements, I in the left-hand column and II in the right-hand column. For each question, decide if statement I is true or false AND whether statement II is true or false, and fill in the corresponding T or F ovals in the answer spaces. *Fill in oval C/E (cause/effect) if and only if statement II is a correct explanation of statement I. I 39. Balanced equations have the same number of reactant atoms the products atoms… 40. The reaction in which HgO is heated to release O2 is called decomposition… 39. 40. I T F T F II T F T F C/E* because… because… II the conservation of matter must apply in all regular chemical equations. in a decomposition reaction, the original compound is broken apart into equal numbers of atoms. APPLICATION PROBLEMS…(Including all tested objectives) Recognize a balanced equation using appropriate symbols, given a word equation Classify a chemical reaction as combination, decomposition, combustion, single replacement, or double replacement. Write a balanced chemical equation for a combustion reaction A confiscated white substance, suspected of being cocaine, was purified by a forensic chemist and subjected to elemental analysis. Combustion of the sample yielded 34 parts carbon dioxide to 21 parts water to 1 part nitrogen. Analysis for nitrogen shows that the ratio between cocaine and nitrogen is always 2:1. The formula of cocaine is C17H21NO4. Can the forensic chemist conclude that the suspected compound is cocaine? Why or why not? SHOW YOUR WORK. Antacids such as Tums or Rolaids use the replacement reaction of calcium carbonate with acid in the stomach to relieve heartburn. To see how this works, use the materials below to observe what happens when calcium carbonate reacts with hydrochloric acid. Materials: - Powder, calcium carbonate - Liquid, 1 M hydrochloric acid - Dropper - Popsicle stick spatula - Rubber gloves - Goggles - Well plate Questions: 1. Describe in words what you see happening. 2. Write a balanced chemical equation for all visible reactions that occur. 3. Explain how this simulates the relief of heartburn in the body.