Coach Coker`s Notes - Coach Coker`s Chemistry
advertisement
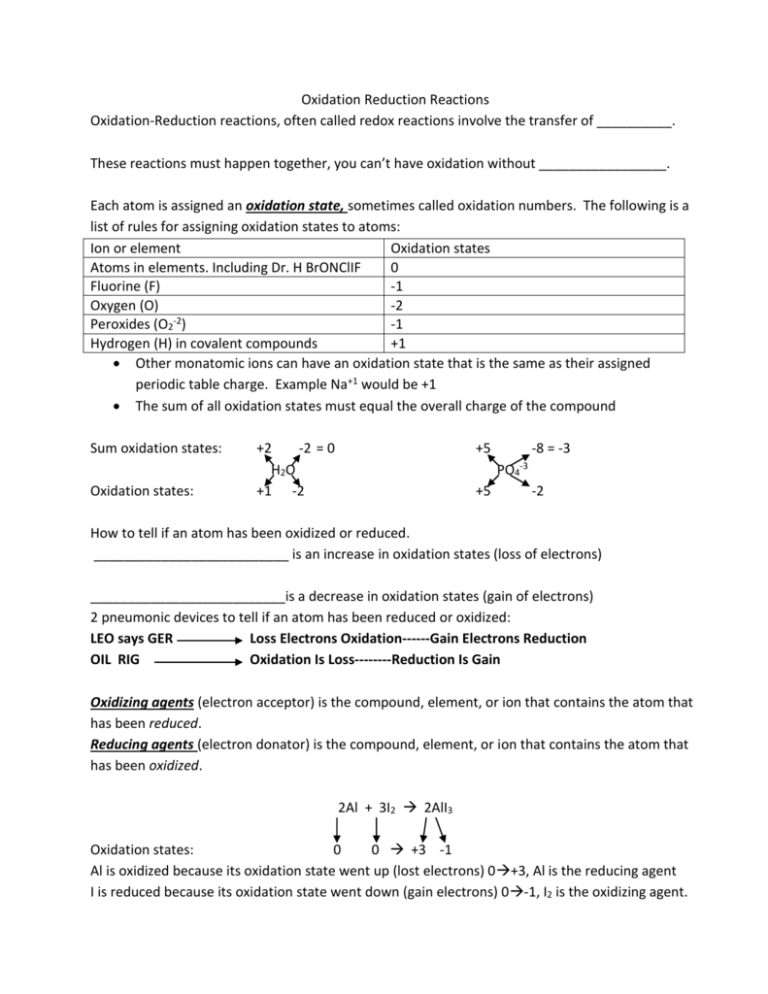
Oxidation Reduction Reactions Oxidation-Reduction reactions, often called redox reactions involve the transfer of __________. These reactions must happen together, you can’t have oxidation without _________________. Each atom is assigned an oxidation state, sometimes called oxidation numbers. The following is a list of rules for assigning oxidation states to atoms: Ion or element Oxidation states Atoms in elements. Including Dr. H BrONClIF 0 Fluorine (F) -1 Oxygen (O) -2 -2 Peroxides (O2 ) -1 Hydrogen (H) in covalent compounds +1 Other monatomic ions can have an oxidation state that is the same as their assigned periodic table charge. Example Na+1 would be +1 The sum of all oxidation states must equal the overall charge of the compound Sum oxidation states: Oxidation states: +2 -2 = 0 H2O +1 -2 +5 -8 = -3 PO4 +5 -3 -2 How to tell if an atom has been oxidized or reduced. __________________________ is an increase in oxidation states (loss of electrons) __________________________is a decrease in oxidation states (gain of electrons) 2 pneumonic devices to tell if an atom has been reduced or oxidized: LEO says GER Loss Electrons Oxidation------Gain Electrons Reduction OIL RIG Oxidation Is Loss--------Reduction Is Gain Oxidizing agents (electron acceptor) is the compound, element, or ion that contains the atom that has been reduced. Reducing agents (electron donator) is the compound, element, or ion that contains the atom that has been oxidized. 2Al + 3I2 2AlI3 Oxidation states: 0 0 +3 -1 Al is oxidized because its oxidation state went up (lost electrons) 0+3, Al is the reducing agent I is reduced because its oxidation state went down (gain electrons) 0-1, I2 is the oxidizing agent. Oxidation states: Balancing Redox Reactions in ACIDIC media H+ + Cr2O72- + C2H5OH Cr3+ + CO2 + H2O +1 +6 -2 -4 +1 -2 +1 +3 +4 -2 +1 -2 Steps in balancing redox reactions in acidic media: 1) Break equation into 2 half reactions: Reduction half reaction and oxidation half reaction. Ignore any H+ or H2O that are in the original reaction. Reduction: Cr2O72- Cr3+ Oxidation: C2H5OH CO2 2) Balance all elements except hydrogen (H) and oxygen (O) Reduction: Cr2O72- 2Cr3+ Oxidation: C2H5OH 2CO2 3) Balance oxygens using H2O Reduction: Cr2O72- 2Cr3+ + 7H2O Oxidation: C2H5OH + 3H2O 2CO2 Balance Hydrogens using H+ Reduction: 14H+ + Cr2O72- 2Cr3+ + 7H2O Oxidation: C2H5OH + 3H2O 2CO2 + 12H+ 4) Balance charges using electrons (e-) Reduction: 14H+ + Cr2O72- + 6e- 2Cr3+ + 7H2O Oxidation: C2H5OH + 3H2O 2CO2 + 12H+ + 12e5) Multiply through one or both equations to make sure electrons will cancel out Reduction: x2) 28H+ + 2Cr2O72- + 12e- 4Cr3+ + 14H2O Oxidation: C2H5OH + 3H2O 2CO2 + 12H+ + 12e6) Finally, combine like terms on same side of and cancel like terms on opposite side of Reduction: 16H+ + 2Cr2O72- + 12e- 4Cr3+ + 11H2O Oxidation: C2H5OH + 3H2O 2CO2 + 12H+ + 12e The electrons completely cancel each other out 3 H2O on oxd half rxn cancel to zero and reduce the waters on the red from 14 to 11 The 12 H+ on the oxd half rxn cancel to zero and reduce the H+ from 28 to 16 on red half 7) Final Balanced redox equation in acidic media: 16H+ + 2Cr2O72- + C2H5OH 4Cr3+ + 11H2O + 2CO2 Note how the total charge on both sides are equal (12+ 12+)