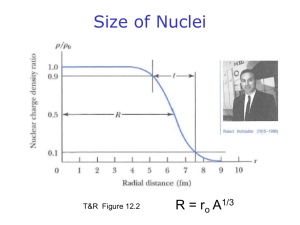
Unit 1 – Atomic Theory and Structure
advertisement
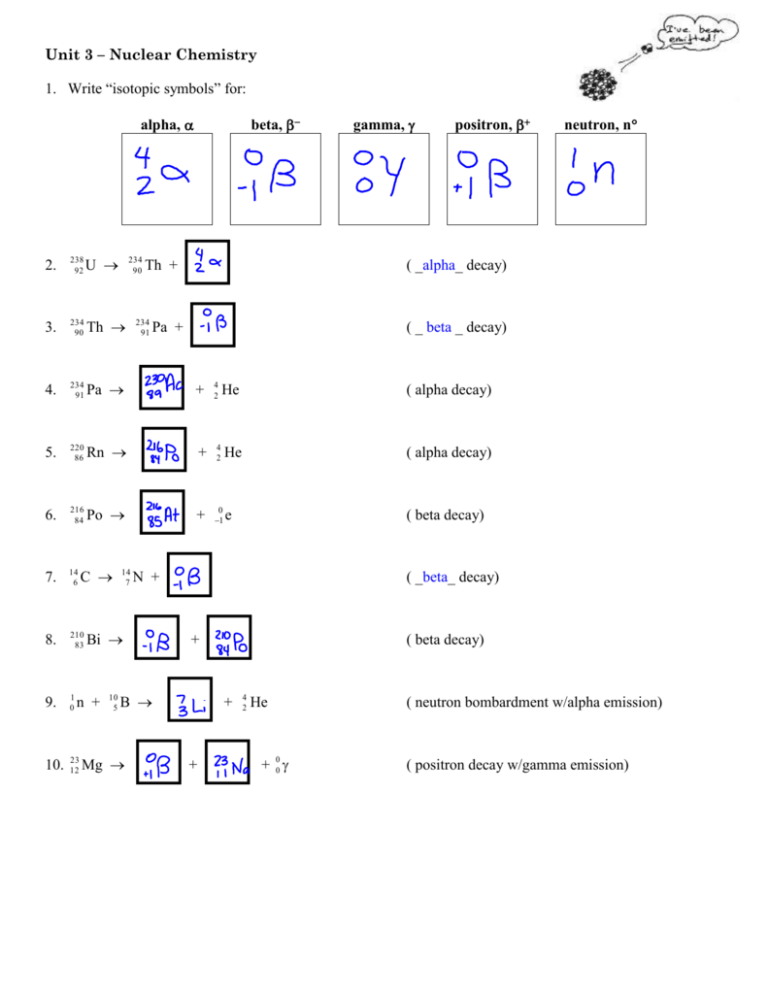
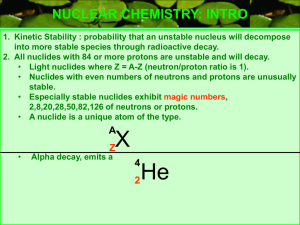
Unit 3 – Nuclear Chemistry 1. Write “isotopic symbols” for: alpha, 2. 238 92 U 3. 234 90 Th 4. 234 91 Pa 5. 220 86 Rn 6. 216 84 Po 7. 14 6 8. 210 83 9. 1 0 10. 23 12 C 234 90 14 7 Bi n + 10 5 Th + 234 91 Pa + beta, positron, ( _ beta _ decay) + 4 2 He ( alpha decay) + 4 2 He ( alpha decay) + 0 1 e ( beta decay) N + ( _beta_ decay) + + + neutron, n ( _alpha_ decay) B Mg gamma, 4 2 ( beta decay) He + 00 ( neutron bombardment w/alpha emission) ( positron decay w/gamma emission) 11. Write equations for the following reactions: a. The production of 56Mn by neutron bombardment of 55Mn. b. The production of the new element dubnium by bombarding elements of nuclei. c. The bombardment of 27 13 Al with particles to produce to silicon by positron emission. 31 15 249 98 Cf with 15 7 N P and the subsequent decay of d. The conversion of potassium-40 to argon-40 by electron capture. e. The production of carbon-14 in the upper atmosphere by neutron bombardment of f. Uranium-238 that undergoes a series of 4 alpha emissions and one beta emission. 12. According to the above data, what is the half-life of the substance? 13. What percent of the original sample remains after 4 hours? 14. Sketch on the graph above, a curve for a substance whose half-life is 2.0 hrs. 15. Iodine-131 has a half-life of 8 days. What percent of a sample remains after 24 days? 13 6 C. 31 15 P