PHYS 212-071
advertisement
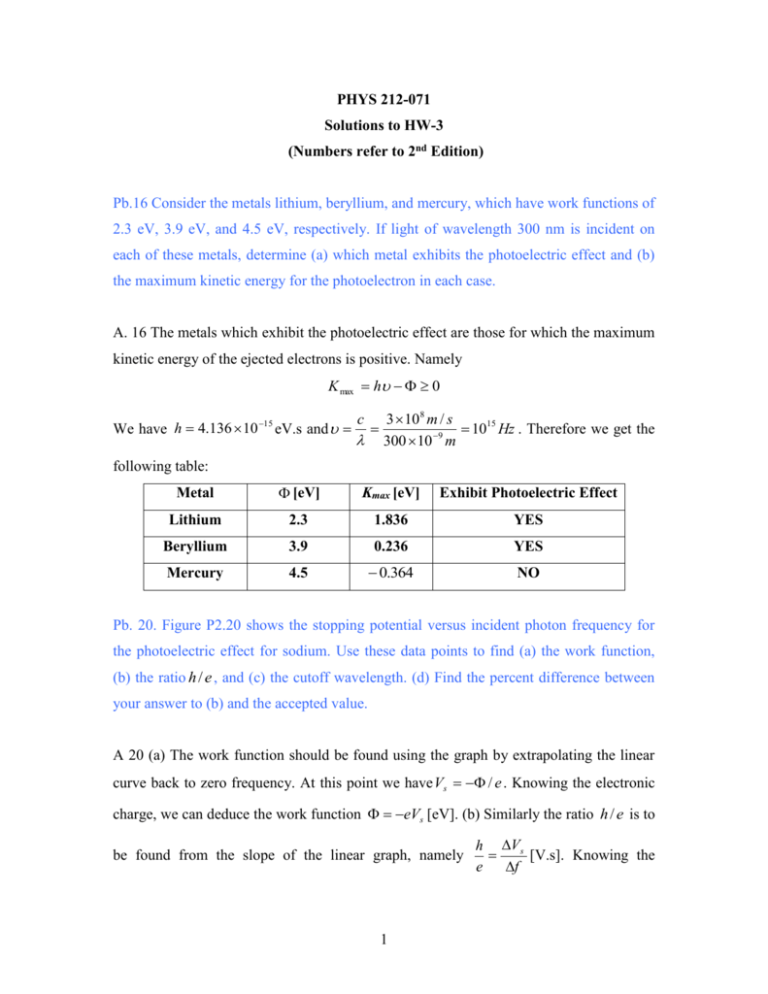
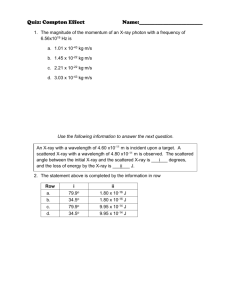
PHYS 212-071 Solutions to HW-3 (Numbers refer to 2nd Edition) Pb.16 Consider the metals lithium, beryllium, and mercury, which have work functions of 2.3 eV, 3.9 eV, and 4.5 eV, respectively. If light of wavelength 300 nm is incident on each of these metals, determine (a) which metal exhibits the photoelectric effect and (b) the maximum kinetic energy for the photoelectron in each case. A. 16 The metals which exhibit the photoelectric effect are those for which the maximum kinetic energy of the ejected electrons is positive. Namely K max h 0 We have h 4.136 10 15 eV.s and c 3 10 8 m / s 1015 Hz . Therefore we get the 300 10 9 m following table: Metal [eV] Kmax [eV] Exhibit Photoelectric Effect Lithium 2.3 1.836 YES Beryllium 3.9 0.236 YES Mercury 4.5 0.364 NO Pb. 20. Figure P2.20 shows the stopping potential versus incident photon frequency for the photoelectric effect for sodium. Use these data points to find (a) the work function, (b) the ratio h / e , and (c) the cutoff wavelength. (d) Find the percent difference between your answer to (b) and the accepted value. A 20 (a) The work function should be found using the graph by extrapolating the linear curve back to zero frequency. At this point we have Vs / e . Knowing the electronic charge, we can deduce the work function eVs [eV]. (b) Similarly the ratio h / e is to be found from the slope of the linear graph, namely 1 h Vs [V.s]. Knowing the e f electronic charge we can find Planck’s constant from the graph h graph . (c) The cutoff wavelength is the wavelength for which K max hf 0 hc 0 0. 0 [nm] hc 1240.8[eV .nm] . [eV ] (d) The % difference can be found by calculating | hgraph haccepted | h [%] 100 h haccepted Pb. 25. X-rays with a wavelength of 0.040 nm undergo Compton scattering. (a) Find the wavelength of photons scattered at angles of 30o, 60o, 120o, 150o, 180o, and 210o. (b) Find the energy of the scattered electrons corresponding to these scattered x-rays. (c) Which one the given scattering angles provides the electron with the greatest energy? A 25. (a) The wavelengths of the scattered photons can be calculated using the Compton scattering formula 0 h 1 cos , where h 0.00243 nm is the me c me c Compton wavelength of the electron. The kinetic energies of the scattered electrons can be found using the following formula K e h 0 h hc 0 hc . We therefore get the following table: [degrees] [nm] [nm] 30 0.000326 0.040326 60 0.001215 0.041215 120 0.003645 0.043645 150 0.004534 0.044534 180 0.00486 0.04486 210 0.004534 0.044534 K e [eV] 250 914 2591 3158 3361 3158 The scattering angle of 180o provides the electron with the greatest kinetic energy of 3.4 keV. 2 Pb. 32. Show that Compton formula h 1 cos me c results when expressions for the electron energy (Equation 2.34) and momentum (Equation 2.35) are substituted into the relativistic energy expression, Ee pe c 2 me c 4 2 2 2 A 32. Equation 2.34 gives the (total) electron energy: Ee hf hf me c 2 . Equation 2 h 2 ff hf hf cos . momentum: p e2 c2 c c 2 (2.35) gives the electron’s 2 By substitution of these expressions into the relativistic energy expression we get: hf hf m c 2 2 e hf 2 hf 2 2h 2 ff 2 2 4 cos c me c 2 c c c Expanding the square on the left-hand side of the equation and after eliminating equal terms we get: f f ff Multiplying through by h 1 cos me c 2 c c c , and recalling that and , we get f ff f h 1 cos me c QED. 3