The frequency dependence of polarization
advertisement

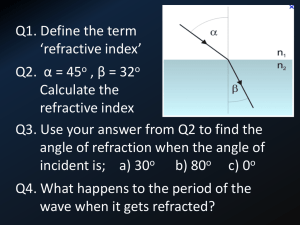
Electric properties of material II The frequency dependence of polarization We distinguish roughly 3 different types of polarization. The total polarization, Pt is at first divided into two Pt Po Pd 1. the orientation and distortion polarization respectively. Orientation refers to the whole molecule, while distortion is an intramolecular event meaning the movement of electrons compared to the atomic frame, and the movement of individual atoms compared to the mass centre of molecule. Therefore, Pt Po Pe Pa 1a. distortion is replaced by electron and atomic polarization. One of the polarizing sources is the electric vector of electromagnetic (EM) waves which generates periodically changing E field like it is shown in Figure 1. below Figure 1. Travelling electromagnetic wave with periodically changing intensity of electric and magnetic field. The vectors are perpendicular to each other. This alternating field is applicable to distiguish different types of polarization. In the wide frequency range of EM waves from radio frequency to ultraviolet radiation may couple to the medium, if the frequency of mechanism is grater El.Prop.02. 1 then the field’s frequency. As the frequency becomes larger, the slower frequency mechanisms drop off. Po orientation polarization A permanent dipole moment is caused by unbalanced sharing of electrons by atoms of a molecule. In an absence of an external electric field, these moments are oriented in a random order such that no net polarization is present. Under an external electric field, the dipoles rotate to align with the electric field causing orientation polarization to occur. The random motion of molecules are set parallel to the alternating field lines by low frequency photons. A dipole in liquid phase follow the periodic change in E of EM waves, if frequency of radiation is lower than 1011 Hz. A molecule in liquid phase takes about 1 ps (period of motion) to rotate in the fluid, the change occurs when measurements are made at frequencies 1011 Hz. When the frequency of radiation exceeds the frequency of rotation orientation polarization is lost. Figure 2. Orientations of non-polar molecules. This type of polarizaton exists only in liquid phase. Figure 3. Dipolar rotation in liquid phase. A circle with an opening angle 2θ relative to the field direction shown. El.Prop.02. 2 Atomic polarization, Pa Electron and atomic polarization are independent of dipole orientation generated by EM waves. The magnitude of Pd is dependent on polarizability. The atoms in the molecule are bent and stretched by the radiation of EM waves, while its dipole is changed. This polarization is called atomic polarization, and its frequency falls in the infrared (1011 – 1014 s-1). A B Figure 4. Symmetric stretching (A) bending motion: scissoring (B) Electron polarization The electron polarization is a property of electrons. They lightly follow the frequency of photons in the visible and ultraviolet range. (1014 – 1017 s-1). Total polarization can be measured in the microwave region, electronic polarization exclusively can be observed in the vis or uv ranges, see Figure 5. below. El.Prop.02. 3 Fig.5. The frequency dependence of polarization. Note: The outer electric field, photons in the IR range capable to increase polarization in ranges where there is no vibrational excitation, i.e. there is no absorbtion band in the IR spectrum. The same is true for electron excitation in the UV VIS ranges. In general, polarization effect can not be obseved purely in ranges where absorption bands can be found. See peaks in Figure 5. Refractive index Association Prisms and gratings are parts of the monochromators in spectrophotometers. They resolute the incoming white light into its component wavelengths producing monochromatic light to the output. The working principle of monochromators (prism, grating) is the dispersion of refractive index. El.Prop.02. 4 Absolute refractive index Figure 6. The reflection and refraction of light at the boundary of two phases. In Figure 6. the upper medium is less denser with refractive index relation nincidence < ntransmitted, . vi, vt and vr are the phase velocities of incident transmitted and reflected beam respectively. When light advances from smaller to higher refractive index phases θi, the angle of incidence, to the normal direction, is greater than that of θt, transmitted beam. Light beam arising from vacuum to a phase boundary refracts and advances in the (optically) denser material a slower phase velocity, v. The ratio of the two velocities proportional to the ratio of material constants called refractive indices vi n t v t ni vi c 3 108 m / s 2. The refractive index of vacuum, ni = 1.000, therefore the velocity ratio gives the absolute refractive index of material. v nt i vt 2a. Owing to inequality vi > vt the value of nt is always greater than one. The refractive index of air is almost identical to that of vacuum, 1,00027 El.Prop.02. 5 Taking into account the angle dependence of incident and transmitted beam another approximation can be applied. Schnell’s law sin θi ni sin θt nt 3. The sine of angle of incidence is inversely proportional to the absolute refractive index of the phase; see the notation in the figure above. We can clearly define the relative refractive index, nr nr nt sin θi ni sin θt 3a. If the light travels from lighter to denser medium and the reference is the lighter medium nr > 1. The phase velocity of incident ray is greater because ni is smaller. Dispersion The refractive index is wavelength and temperature dependent, its wavelength dependence is the dispersion. Figure 7. In a dispersive prism, material dispersion (a wavelength-dependent refractive index) causes different colors to refract at different angles, splitting white light into a rainbow. The difference in deviation of blue and red for a particular prism is the measure of dispersion. El.Prop.02. 6 Owing to its wavelength dependence, the refractive index of different materials is measured at a certain frequency of visible light (monochromatic light), e.g. at the sodium D-line. The frequency of this emission line is c 3 108 ms 1 Na, D 589 nm 5,09 1014 s-1 -9 Na, D 589 10 m Refractive index of liquids is easy to determine by using an Abbe type refractometer, which commonly applies sodium D-line as monochromatic source. By using Maxwell’s law for nonmagnetic materials nr2 εr 4. refractive index is used for determination molar polarization, polarizability. This equality, Eq. 4. is substituted to Debye equation, we get n r2 1 N Pm 2 Vm A 3 0 nr 2 2 3 kT 5. If μ is zero, there is no orientation polarization, and we get nr2 1 N Rm 2 Vm A 30 nr 2 5a. the result is called Clausius Mossotti equation, and Rm is molar refraction. The polarizability can separately be determined by this equation. Refractive index of liquids is easy to determine by using an Abbe type refractometer, which commonly applies sodium D-line as monochromatic source. El.Prop.02. 7 The energy of molecular interactions In an aqueous salt solution we distinguish ion-ion, ion-dipole and dipole-dipole interactions. The characterization of different interactions can be described by potential energy vs. distance functions. Ion – dipole interaction. The strength of interaction of charged particles can be given either by force or potential energy. We choose potential energy, V for characterization. V 1 4 0 qA qB r 1. where r is the distance between qA and qB. The potential energy of interaction, of an ion dipole system is the sum of potential energies in pairs. Figure 1. A point charge q2 is placed at a distance r from the centre of dipole with charge separation l. This system contains three charges q1,–q1 and q2 and three interactions: a repulsive between q1 ↔ q2, an attractive between -q1 ↔ q2. We do not consider the potential energy of dipole itself. We have to take into account all the potential energy of interactions by pairs and simply add them to obtain the net energy of interaction of system. El.Prop.02. 8 From Figure 1. we get: The dipole has a moment: μ1 = q1 l l 2 l the distance between -q1 and q2 is: r . 2 the distance between +q1 and q2 is: r For repulsive interaction: Vr 1 4 0 q1 q2 l r 2 2. For attractive interaction: Va 1 4 0 q1 q2 l r 2 2a. The potential energy of interaction between ion and dipole is the sum of attractive and repulsive interactions between them, q1q2 1 1 V Vr Va 4 0 r l r l 2 2 Applying simplifications: finding the common denominator and multiplying the denominator part by r/r q1q2 1 1 q1q2 1 1 V 4 0 2r l 2r l 4 0 r 2r l r 2r l 2 2 2r 2r El.Prop.02. 9 q1q2 1 1 V l l 4 0 r 1 2 r r 1 2 r Let l/2r denote by x V q1q2 1 1 4r0 1 x 1 x We recognize the form to be a series. Expanding the term 1 1 1 x 1 x 1 1 1 x x 2 ... 1 x x 2 ... 1 x 1 x omitting from x2, the sum is 2 x V 2r r 2l l q1q2 qq l 2x 1 2 4r0 4r0 r The dipole moment was formerly defined as μ1 = q1 l, so finally we get V El.Prop.02. q21 4r 2 0 3. 10 The interaction energy decreases more rapidly than that between two monovalent ion. Table 1. Interaction potential energies Interaction type Function Ion-ion Ion-dipole Dipole-dipole London (dispersion) Distance dependence of V 1/r 1 q1q2 V 4 0 r 1/r2 q V 2 21 4r 0 1/r3 2 V 23 1 4r 0 1/r6 Typical energy kJ/mol 250 The smaller the energy of interaction, the shorter the distance where V approximates zero and interactions can be neglected. El.Prop.02. 11 15 2 2