Balancing Equations Notes

Balancing Equations
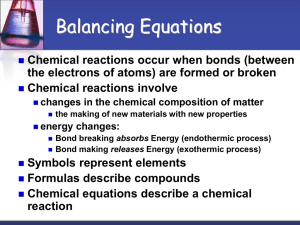
Chemical Equation : a way to represent chemical reactions on paper.
Animation http://www.chemistry.ohio-state.edu/betha/nealChemBal/
The law of conservation of matter states matter cannot be created or destroyed but can change from one form to another.
Therefore, equations must be balanced!
Atoms can be neither created nor destroyed in an ordinary chemical reaction, so there must be the same number of atoms on both sides of the equation.
These numbers are found in a chemical equation:
Subscripts - The small numbers to the lower right of chemical symbols. Subscripts represent the number of atoms of each element in the molecule.
How many atoms of each element are there in one formula unit of ammonium sulfide?
Ammonium sulfide is (NH
4
)
2
S
How many atoms of each element are there in one formula unit of barium nitrate?
Barium nitrate is Ba(NO
3
)
2
Coefficients - The large numbers in front of chemical formulas.
Coefficients represent the number of molecules of the substance in the reaction.
How many atoms of each element are there in three formula units of barium nitrate?
3Ba(NO
3
)
2
Check for Diatomic Molecules - H
2
- N
2
- O
2
- F
2
- Cl
2
- Br
2
- I
2
If these elements appear by themselves in an equation, they must be written with the subscript
2
Please write these diatomic molecules on you blue ion sheet.
Chemical equations give information in two major areas.
First they tell us what substances are reacting and what substances are being produced.
Reactants
Products
What are the reactants?
2H
2
+ O
2
2H
2
O
What are the products?
Second, the coefficients of a balanced equation tell us in what ratio MOLE the substances react or are produced.
2H
2
+ O
2
2H
2
O
How many moles of water are produced?
How many moles of hydrogen are needed?
How many moles of oxygen are needed?
How do we balance equations?
Balance equations by changing coefficients
Never by changing formula subscripts
Steps:
1.
Write all reactants on the left and all products on the right side of the equation arrow. Make sure you write the correct formula for each element.
2.
Use coefficients in front of each formula to balance the number of atoms on each side.
3.
Multiply the coefficient of each element by the subscript of the element to count the atoms. Then list the number of atoms of each element on each side.
4.
It is often easiest to start balancing with an element that appears only once on each side of the arrow. These elements must have the same coefficient. Next balance elements that appear only once on each side but have different numbers of atoms. Finally balance elements that are in two formulas in the same side.
Balance these equations:
_____Zn + ______HCl
_____ZnCl
2
+ _____H
2
_____KClO
3
_____KCl + _____O
2
_____S
8
+ _____F
2
_____SF
6
_____Fe + _____O
2
_____Fe
2
O
3
_____C
2
H
6
+ _____O
2
_____CO
2
+ _____H
2
O
More Practice http://www.fordhamprep.com/gcurran/sho/sho/worksheets/worksht81b.ht
m