Answer-key--Atomic-Nuclear
advertisement
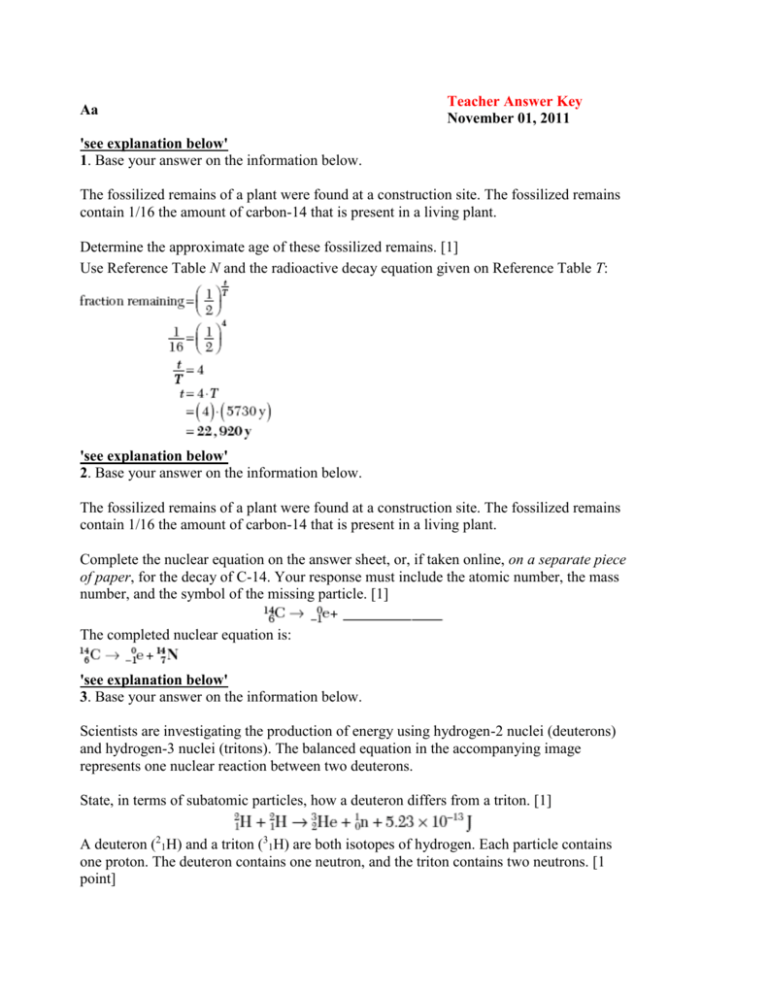
Aa Teacher Answer Key November 01, 2011 'see explanation below' 1. Base your answer on the information below. The fossilized remains of a plant were found at a construction site. The fossilized remains contain 1/16 the amount of carbon-14 that is present in a living plant. Determine the approximate age of these fossilized remains. [1] Use Reference Table N and the radioactive decay equation given on Reference Table T: 'see explanation below' 2. Base your answer on the information below. The fossilized remains of a plant were found at a construction site. The fossilized remains contain 1/16 the amount of carbon-14 that is present in a living plant. Complete the nuclear equation on the answer sheet, or, if taken online, on a separate piece of paper, for the decay of C-14. Your response must include the atomic number, the mass number, and the symbol of the missing particle. [1] The completed nuclear equation is: 'see explanation below' 3. Base your answer on the information below. Scientists are investigating the production of energy using hydrogen-2 nuclei (deuterons) and hydrogen-3 nuclei (tritons). The balanced equation in the accompanying image represents one nuclear reaction between two deuterons. State, in terms of subatomic particles, how a deuteron differs from a triton. [1] A deuteron (21H) and a triton (31H) are both isotopes of hydrogen. Each particle contains one proton. The deuteron contains one neutron, and the triton contains two neutrons. [1 point] 'see explanation below' 4. Base your answer on the information below. Scientists are investigating the production of energy using hydrogen-2 nuclei (deuterons) and hydrogen-3 nuclei (tritons). The balanced equation in the accompanying image represents one nuclear reaction between two deuterons. Identify the type of nuclear reaction represented by the equation. [1] A nuclear fusion reaction is one in which light nuclei combine to form heavier nuclei. [1 point] 'see explanation below' 5. Base your answer on the information below. The radioisotope uranium-238 occurs naturally in Earth's crust. The disintegration of this radioisotope is the first in a series of spontaneous decays. The sixth decay in this series produces the radioisotope radon-222. The decay of radon222 produces the radioisotope polonium-218 that has a half life of 3.04 minutes. Eventually, the stable isotope lead-206 is produced by the alpha decay of an unstable nuclide. Determine the original mass of a sample of Po-218, if 0.50 milligram of the sample remains unchanged after 12.16 minutes. [1] Use the radioactive decay equation given on Reference Table T: (see image) [1 point] 'see explanation below' 6. Base your answer on the information below. The radioisotope uranium-238 occurs naturally in Earth's crust. The disintegration of this radioisotope is the first in a series of spontaneous decays. The sixth decay in this series produces the radioisotope radon-222. The decay of radon222 produces the radioisotope polonium-218 that has a half life of 3.04 minutes. Eventually, the stable isotope lead-206 is produced by the alpha decay of an unstable nuclide. Complete the nuclear equation shown on the accompanying diagram for the decay of the unstable nuclide that produces Pb-206, by writing a notation for the missing nuclide. [1] An alpha particle is the nucleus of a helium-4 atom. A nuclear equation is balanced if the sum of the atomic numbers and the sum of the mass numbers are equal on both sides of the arrow. The complete nuclear equation is shown in the accompanying image: The missing nuclide in the equation is Po-210. [1 point] 'see explanation below' 7. Write an electron configuration for an atom of aluminum-27 in an excited state. [1] Refer to the Periodic Table of the Elements. The ground-state electron configuration of any isotope of aluminum (Al) is 2-8-3. An excited atom is one in which one or more electrons have been promoted to higher shells. Remember: The first shell may contain no more than 2 electrons, and the second shell may contain no more than 8. Some acceptable examples are: 2-7-4 1-8-4 2-6-2-3 'see explanation below' 8. Base your answer on the information below. The accepted values for the atomic mass and percent natural abundance of each naturally occurring isotope of silicon are given in the accompanying data table. Determine the total number of neutrons in an atom of Si-29. [1] Refer to the Periodic Table of the Elements. The atomic number of an atom of silicon (Si) is 14 and is equal to the number of protons in the nucleus of the atom. The mass number of the isotope Si-29 is 29 and is equal to the sum of the number of protons and neutrons in its nucleus. Therefore the number of neutrons is equal to 29 – 14 = 15. 'see explanation below' 9. Base your answer on the information below. The accepted values for the atomic mass and percent natural abundance of each naturally occurring isotope of silicon are given in the accompanying data table. In the space provided, or, if taken online, on a separate piece of paper, show a correct numerical setup for calculating the atomic mass of Si. [1] To calculate the (average) atomic mass of the element Si, multiply the atomic mass of each isotope by the decimal equivalent of the percent natural abundance, as shown below: (average) atomic mass = (27.98) · (0.922)+(28.98) · (0.0469)+(29.97) · (0.0309) 'see explanation below' 10. Base your answer on the information below. Elements with atomic numbers 112 and 114 have been produced and their IUPAC names are pending approval. However, an element that would be put between these two elements on the Periodic Table has not yet been produced. If produced, this element will be identified by the symbol Uut until an IUPAC name is approved. Determine the charge of an Uut nucleus. Your response must include both the numerical value and the sign of the charge. [1] The atomic number of Uut is 113. Therefore, the nuclear charge is +113. 'see explanation below' 11. Base your answer on the information below. In a laboratory, a glass tube is filled with hydrogen gas at a very low pressure. When a scientist applies high voltage between metal electrodes in the tube, light is emitted. The scientist analyzes the light with a spectroscope and observes four distinct spectral lines. The accompanying table gives the color, frequency, and energy for each of the four spectral lines. The unit for frequency is hertz, Hz. Explain, in terms of subatomic particles and energy states, why light is emitted by the hydrogen gas. [1] Light is emitted when a hydrogen atom changes from a higher to a lower energy state. This change corresponds to an electron dropping from a higher electron shell to a lower one. [1 point] 'see explanation below' 12. Base your answer on the information in the accompanying table. State, in terms of subatomic particles, how an atom of Cu-63 differs from an atom of Cu65. [1] Refer to the Periodic Table of the Elements. Since both atoms are copper (Cu), both have 29 protons in their nuclei. The mass number of an atom is the sum of the protons and neutrons in its nucleus. An atom of Cu-63 has 63 – 29 or 34 neutrons in its nucleus. An atom of Cu-65 has 65 – 29 or 36 neutrons in its nucleus. Therefore, an atom of Cu-63 has fewer neutrons than an atom of Cu-65. [1 point] 'see explanation below' 13. Base your answer on the information in the accompanying table. What is the total number of electrons in an atom of Cu-65? [1] An atom is a neutral particle. The number of protons must be equal to the number of electrons. Since copper contains 29 protons, it also contains 29 electrons. [1 point] 'see explanation below' 14. Base your answer on the information in the accompanying table. In the space on the answer sheet or on a separate piece of paper, show a correct numerical setup for calculating the atomic mass of copper. [1] To calculate the (average) atomic mass of the element Cu, multiply the atomic mass of each isotope by the decimal equivalent of the percent natural abundance, as shown below: (average) atomic mass = (62.930) · (0.6917) + (64.928) · (0.3083) [1 point] 'see explanation below' 15. Describe the electrons in an atom of carbon in the ground state. Your response must include: the charge of an electron [1] the location of electrons based on the wave-mechanical model [1] the total number of electrons in a carbon atom [1] An electron has a negative charge. According to the wave-mechanical model, electrons are located in orbitals (or regions of most probable location). An atom of carbon has a total of 6 electrons. One point is awarded for each acceptable response. [3 points] 'see explanation below' 16. On a separate piece of paper, write an electron configuration for a silicon atom in an excited state. [1] Refer to the Periodic Table of the Elements. The ground-state electron configuration for an atom of silicon (Si, atomic number 14) is 2–8–4. An excited atom has one or more of its electrons promoted to a higher energy level. Many different configurations are possible, including 2-7-5, 1-8-5, and 2-8-3-1. Note that only one configuration needs to be given in order to receive credit. [1 point] 'see explanation below' 17. Base your answer on the information below. In the gold foil experiment, a thin sheet of gold was bombarded with alpha particles. Almost all the alpha particles passed straight through the foil. Only a few alpha particles were deflected from their original paths. State one conclusion about atomic structure based on the observation that almost all alpha particles passed straight through the foil. [1] Since most of the alpha particles passed through without deflection, Rutherford concluded that atoms consist mostly of empty space. [1 point] 'see explanation below' 18. Base your answer on the information below. In the gold foil experiment, a thin sheet of gold was bombarded with alpha particles. Almost all the alpha particles passed straight through the foil. Only a few alpha particles were deflected from their original paths. Explain, in terms of charged particles, why some of the alpha particles were deflected. [1] Alpha particles are positive, and a few were repelled by the positively charged nucleus. [1 point] 2 19. What is the decay mode of 37K? 2 See Reference Table N. The nuclide 37K decays to produce a β+ particle. 1. alpha particle 2. beta particle 3 The penetrating powers of the nuclear emissions given in decreasing order are gamma radiation > beta particle ≈ positron > alpha particle. 1. 1 2. 2 3 20. Which nuclear emission has the greatest penetrating power? 3. gamma radiation 4. positron 4 21. What is the mass number of an alpha particle? 3. 0 4. 4 4 See Reference Table O. The mass number of a particle is the sum of the protons and 4 neutrons contained within the nucleus of the 22. Which nuclear equation represents a natural particle. An alpha particle is a helium-4 transmutation? nucleus; its mass number is 4. 4 Natural transmutation reactions occur spontaneously and without the presence of a bombarding particle. Of the choices given only choice (4), 23592U→23190Th + 42He, meets this criterion. 1. form heavy nuclides from light nuclides 2. form light nuclides from heavy nuclides 3 In both nuclear fission and nuclear fusion reactions, significant amounts of mass are converted into large amounts of energy (according to Einstein's famous E = mc 2 equation). 1. carbon-14, treatment of cancer 3 23. A nuclear fission reaction and a nuclear fusion reaction are similar because both reactions 3. release a large amount of energy 4. absorb a large amount of energy 3 24. Which nuclide is paired with a specific use of that nuclide? 3. iodine-131, treatment of thyroid disorders 2. cobalt-60, dating of rock formations 4. uranium-238, dating of once-living organisms 3 Iodine is concentrated in thyroid tissue. Therefore, iodine-131 is used to treat thyroid disorders. Wrong Choices Explained: (1) Carbon-14 is used in the dating of onceliving organisms. (2) Cobalt-60 is used in the treatment of certain types of cancer. (4) Uranium-238 is used in the dating of rock formations. 1. 1.04 s 2. 2.08 s 3 Use the radioactive decay formula given on Reference Table T. First, determine the number of half-life periods t/T in 8.32 seconds. Then use this value to determine the half-life of the isotope: 1. addition 2. fission 3 25. An original sample of the radioisotope fluorine-21 had a mass of 80.0 milligrams. Only 20.0 milligrams of this original sample remain unchanged after 8.32 seconds. What is the half-life of fluorine-21? 3. 4.16 s 4. 8.32 s 3 26. A nuclear reaction in which two light nuclei combine to form a more massive nucleus is called 3. fusion 4. substitution 3 The nuclear process of combining two light nuclei in a single, more massive nucleus is known as fusion. Wrong Choices Explained: (1), (4) Addition and substitution are organic reactions, not nuclear processes. (2) In nuclear fission, a single heavy nucleus is split into less massive fragments. 1. absorb electrons 2. absorb protons 3 See Reference Table N. Spontaneous decay is the process by which unstable radioactive nuclei eventually achieve stability. The spontaneous decay of 226Ra produces alpha particles. 1. acid rain 2. helium gas 3 27. The nucleus of a radium-226 atom is unstable, which causes the nucleus to spontaneously 3. decay 4. oxidize 4 28. A serious risk factor associated with the operation of a nuclear power plant is the production of 3. greenhouse gases, such as CO2 4. radioisotopes with long half-lives 4 A number of problems are associated with 4 nuclear power facilities. One of the main 29. Given the balanced equation representing a nuclear problems is the disposal and storage of radioactive isotopes with long half-lives. reaction: Which particle is represented by X ? 4 In a balanced nuclear equation, the sum of the atomic numbers and the sum of the mass numbers on both sides of the equation must be equal. The sum of the atomic numbers on both sides of this equation total 92. Therefore, the atomic number (nuclear 4 charge) of X must be 0. The mass numbers 30. A change in the nucleus of an atom that converts of this equation total 236 on the left side and the atom from one element to another element is called 233 on the right side. The difference of 3 is carried by the three X particles. Therefore, the mass number of each X is 1. In summary, the symbol for X is 10X. Use Reference Table O to identify the particle as a neutron (10n). 1. combustion 3. polymerization 2. neutralization 4. transmutation 4 Transmutation is defined as the change in 2 the nucleus of an atom that converts the 31. Which particle is emitted from a hydrogen-3 atom from one element to another. nucleus when it undergoes radioactive decay? 2 See Reference Tables N and O. The radioisotope hydrogen-3 (3H) emits beta particles as it decays. 1. 2.87 d 2. 3.82 d 2 Use the radioactive decay equation on Reference Table T: (see image) 1. decomposition 2. neutralization 2 32. What is the half-life of a radioisotope if 25.0 grams of an original 200.-gram sample of the isotope remains unchanged after 11.46 days? 3. 11.46 d 4. 34.38 d 4 33. In which type of reaction is an atom of one element converted to an atom of a different element? 3. saponification 4. transmutation 4 Transmutation is defined as the nuclear 2 process in which the nucleus of one element 34. Which nuclide is listed with its half-life and decay is transformed into the nucleus of another element. 2 Use Reference Table N. Of the choices given, only choice (2), N–16, 7.2 s, β-, lists both the correct half-life and correct decay mode for the given nuclide. 1. C-14 2. U-238 mode? 3 35. Which isotope is used to treat cancer? 3. Co-60 4. Pb-206 3 Radioactive isotopes have many uses in science, medicine, and industry. The isotope Co-60 is widely used to treat cancer by radiation. Wrong Choices Explained: (1) The isotope C-14 is used to determine the date of once-living materials, and to trace the path of carbon in organic and biochemical reactions. (2) The isotope U-238 is used to determine the age of rocks and also to "breed" nuclear fuel. (4) The isotope Pb-206 is not radioactive. 1. number of protons equals the number of electrons 2. number of protons equals the number of neutrons 1 Protons and electrons have equal but opposite charges, and neutrons have no charge. An atom is neutral because the number of protons equals the number of electrons. 1. In the third shell, an electron has more energy and is closer to the nucleus. 2. In the third shell, an electron has more energy and is farther from the nucleus. 1 36. An atom is electrically neutral because the 3. ratio of the number of neutrons to the number of electrons is 1:1 4. ratio of the number of neutrons to the number of protons is 2:1 2 37. How do the energy and the most probable location of an electron in the third shell of an atom compare to the energy and the most probable location of an electron in the first shell of the same atom? 3. In the third shell, an electron has less energy and is closer to the nucleus. 4. In the third shell, an electron has less energy and is farther from the nucleus. 2 As a shell number increases, the energy of 3 an electron occupying the shell increases 38. Which two particles make up most of the mass of a and the probability is that the electron will hydrogen-2 atom? be located farther from the nucleus. 1. electron and neutron 2. electron and proton 3. proton and neutron 4. proton and positron 3 The nucleus contains most of the mass of 2 an atom. The nucleus of a hydrogen-2 atom 39. What was concluded about the structure of the contains 1 proton and 1 neutron. atom as the result of the gold foil experiment? 1. A positively charged nucleus is 3. A negatively charged nucleus is surrounded by positively charged particles. surrounded by positively charged particles. 2. A positively charged nucleus is 4. A negatively charged nucleus is surrounded by mostly empty space. surrounded by mostly empty space. 2 An English physicist, Ernest Rutherford, beamed alpha particles at thin gold foils. When he examined the scattering patterns of the alpha particles, he concluded that most of the volume of the atom was empty space and that most of the mass of the atom was concentrated in a dense, positively charged nucleus. 1. protons 2. positrons 4 According to the wave-mechanical model of the atom, locating the exact position of an electron around the nucleus is not possible. One can calculate only the probability that an electron will most likely be found within a certain region in space. These regions are called orbitals. 1. a positively charged electron cloud surrounding a positively charged nucleus 2. a positively charged electron cloud surrounding a negatively charged nucleus 4 40. In the wave-mechanical model of the atom, orbitals are regions of the most probable locations of 3. neutrons 4. electrons 3 41. Which phrase describes an atom? 3. a negatively charged electron cloud surrounding a positively charged nucleus 4. a negatively charged electron cloud surrounding a negatively charged nucleus 3 An atom is a neutral particle that contains equal numbers of protons and electrons. The protons are located within the positively 1 charged nucleus, and the negatively charged 42. Which total mass is the smallest? electrons are located in a cloud that surrounds the nucleus. 3. the mass of 1 electron plus the mass of 1 1. the mass of 2 electrons proton 4. the mass of 1 neutron plus the mass of 1 2. the mass of 2 neutrons electron approximate mass of 1 atomic mass unit. The mass of an electron is approximately 0.0005 atomic mass unit. Therefore, of the choices given, the mass of 2 electrons is the smallest. 1. 69 2. 79 2 43. What is the total number of protons in an atom with the electron configuration 2-8-18-32-18-1? 3. 118 4. 197 2 An atom is a neutral particle containing an equal number of protons and electrons. The 4 sum of the number of electrons in the 44. Which particle has the least mass? configuration 2-8-18- 32-18-1 adds to 79; this is also the number of protons present. 4 Use Reference Table O. Of the choices given, choice (4), 0 -1He, which is an electron, is the least massive particle. Wrong Choices Explained: (1) The symbol 4 2He represents an alpha particle, which is approximately 8,000 times 3 45. What information is necessary to determine the more massive than an electron. atomic mass of the element chlorine? (2) The symbol 1 1H represents a proton, which is approximately 2,000 times more massive than an electron. (3) The symbol 1 0n represents a neutron, which is approximately 2,000 times more massive than an electron. 3. the atomic mass and the relative 1. the atomic mass of each artificially abundance of each naturally occurring produced isotope of chlorine, only isotope of chlorine 4. the atomic mass and the relative 2. the relative abundance of each naturally abundance of each naturally occurring and occurring isotope of chlorine, only artificially produced isotope of chlorine 3 By definition, the atomic mass of an element is the weighted average of the element's naturally occurring isotopes. In order to calculate such an average, the mass and relative abundance of each isotope must be known. 1. equals the number of electrons 2. equals the number of neutrons 1 46. In an atom of argon-40, the number of protons 3. is less than the number of electrons 4. is greater than the number of electrons 1 The mass number of an isotope is the sum 1 of the number of protons and neutrons contained within its nucleus. 1. absorbing energy 2. releasing energy 47. An electron in a sodium atom moves from the third shell to the fourth shell. This change is a result of the atom 3. gaining an electron 4. losing an electron 1 By definition, an atom is a neutral particle 2 in which the numbers of protons and 48. Which electron configuration represents an excited electrons are equal. state for a potassium atom? 1. 2-8-7-1 3. 2-8-8-1 2. 2-8-7-2 4. 2-8-8-2 2 Use the Periodic Table of the Elements. Each element is accompanied by its groundstate electron configuration. Since an atom is a neutral particle, the number of electrons in 2 an electron configuration is also the atomic 49. Given the bright-line spectra of three elements and number of the element. An atom in an the spectrum of a mixture formed from at least two of excited state does not match its ground-state these elements: configuration because one or more electrons have been promoted to higher energy levels. Which elements are present in this mixture? In choice (2), 2-8-7-2, an electron in the third energy level of the atom has been promoted to the fourth energy level. 1. E and D, only 2. E and G, only 3. D and G, only 4. D, E, and G 1 To determine the composition of the mixture, its spectrum must be compared with the spectra of elements D, E, and G. All 2 of the spectral lines of elements D and E 50. The greatest composition by mass in an atom of appear in the spectrum of the mixture, but 17 8O is due to the total mass of its only one spectral line of element G is present in the mixture. Therefore, the mixture contains only elements D and E. 1. electrons 3. positrons 2. neutrons 4. protons neutrons, and 8 electrons. The masses of these subatomic particles in decreasing order 2 are mneutron > mproton >> melectron. Since the 51. The accompanying table shows the number of most massive particle (the neutron) is subatomic particles in atom X and in atom Z. present in the greatest quantity (9), it follows that neutrons contribute most to the mass Atom X and atom Z are isotopes of the element composition of 17O. 1. aluminum 2. carbon 2 An element is defined by the number of protons contained in each atom of the element, which is its atomic number. Refer to the Periodic Table of the Elements. The element carbon (C) has an atomic number of 6. 1. 8 2. 2 2 Valence electrons are those found in the outermost shell of an atom of ion. Refer to the Periodic Table of the Elements. The ground-state electron configuration of calcium (Ca, atomic number 20) is 2–8–8–2. There are 2 electrons in the outermost shell of this atom in the ground state. 1. electrons and neutrons 2. electrons and protons 3. magnesium 4. nitrogen 2 52. What is the total number of valence electrons in a calcium atom in the ground state? 3. 18 4. 20 3 53. Which subatomic particles are located in the nucleus of an He-4 atom? 3. neutrons and protons 4. neutrons, protons, and electrons 3 The nuclei of all atoms (with the exception 1 54. In the late 1800s, experiments using cathode ray of H–1) contain protons and neutrons. tubes led to the discovery of the 1. electron 3. positron 2. neutron 4. proton 1 The physicist J. J. Thomson used cathode 4 rays to identify the properties of the negative 55. The atomic mass of titanium is 47.88 atomic mass particles known as electrons. units. This atomic mass represents the 1. total mass of all the protons and neutrons 3. weighted average mass of the most in an atom of Ti abundant isotope of Ti 2. total mass of all the protons, neutrons, and 4. weighted average mass of all the naturally electrons in an atom of Ti occurring isotopes of Ti 4 The average atomic mass of an element is calculated by determining the weighted average of all of the element's naturally occurring isotopes. The most abundant, naturally occurring isotopes have the greatest effect on the value of the average atomic mass. 1. decay mode 2. bright-line spectrum 2 The isotopes K-37 and K-42 are isotopes of the same element, potassium. Each element has a unique bright-line spectrum. Therefore, K-37 and K-42 will have the same bright-line spectrum. Wrong Choices Explained: (1) Use Reference Table N. The decay mode of K-37 is β+; the decay mode of K-42 is β-. (3) The mass number of K-37 is 37, and the mass number of K-42 is 42. (4) An atom of K-37 has 18 neutrons in its nucleus, while an atom of K-42 has 23 neutrons in its nucleus. 2 56. The isotopes K-37 and K-42 have the same 3. mass number for their atoms 4. total number of neutrons in their atoms









