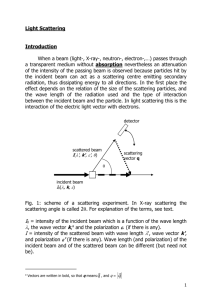
Small angle X-ray scattering from folded and denatured proteins
Protein Structure and Folding
Small angle X-ray scattering from folded and denatured proteins
Small angle X-ray scattering will give information of molecular size and shape. It turns out that the scattering from coillike molecules is conveniently examined from a plot referred to as the Karatky-plot. The purpose of this lecture is to derive scattered intensity to make this plot.
1.
Scattering from a molecule
Consider a molecule with N atoms. The structure factor is the sum over all atomic structure factors taking into account the phases due to the angle between the scattering vector S and vector r n
to the atom n .
F m
( S )
n
N
1 e
2
iS
r n f n
( S )
Intensity is, as usual, the scattering factor squared
I ( S )
F n
F m
*
f o
2 ( S ) n
N
1 e
2
iS
r n m
N
1 e
2
iS
r m
f o
2 ( S ) n
N
1 e
2
iS
r n m where for the simplicity we have neglected any difference between the various atoms. More precisely N equals here the number of electrons from which the scattering takes place anyway. As apparent from the equation the scattering is governed by the pair wise interatomic distances r nm
.
2.
Scattering from a well-defined molecule in a solution
We proceed to calculate the scattered intensity in solution i.e. averaged over all molecular orientations that the molecule tumbles in the solution.
I ( S )
f o
2
( S )
N
2
N n
1 m
1 o o e
2
iS
r n m d
sin f o
2
( S )
N N
2
n
1 m
1 o d
o e
2
iSr n m cos
sin
d
d
4
f o
2
( S ) n
N N
1 m
1
sin( 2
Sr nm
2
Sr nm
)
It is useful to examine the scattering at very small angles in the limit
0 where sin x = x - x 3 /3!+ x 5 /5!- …to obtain
I ( S )
4
f o
2
( S )
N N
1 m
1 n
sin( 2
Sr nm
2
Sr nm
)
4
f o
2
( 0 ) n
N N
1 m
1
1
( 2
Sr nm
)
2
6
The first term in the sum equals the number of electrons squared N 2 and the second can be expressed in terms of radius of gyration
1
R
G
2
1
N
2 n
N
m
2 r nm
1
2 N
2
N n
N m
2 r nm to result in
I ( S )
4
f o
2
( S ) N
2
1
4
2
S
2
R
G
2
/ 3
This is a useful formula because the plot I vs. S 2 will give R
G
. It is even more convenient to apply the approximation exp(x 2 / a ) = 1x 2 / a to allow plotting ln
I ( S )
I ( 0 )
4
2
S
2
R
G
2
/ 3
vs. S 2 to give a straight line proportional to the R
G
2 i.e. the size of the molecule. The plot is referred to as Guinier plot.
It is of interest to examine also the tail region and to note that at large angles I is proportional to 1/ S 4 known as Porod law. This law is derived by examining surface and volume contributions to scattering. It turns out that only structure near the surface will contribute to the scattering at large angles, not the overall large-scale properties. Therefore the
Porod law holds for objects with a well-defined shape to have a surface e.g. folded proteins.
3.
Scattering from a flexible molecule in a solution
The derivation of scattering for a denatured protein has to include also the averaging due to varying inter atomic distances r nm
I ( S )
4
f o
2
( S ) n
N N
1 m
1
sin( 2
Sr nm
2
Sr nm
)
A priori we do not know how the distances are distributed in the denatured ensemble, thus the problem can be very difficult in general. However once we assume a distance distribution W ( r ij
) e.g. that of a random coil we are able to proceed in calculating the average as usual by integration over the probabilities sin( 2
Sr nm
2
Sr nm
)
0
W ( r nm
) sin( 2
Sr nm
2
Sr nm
) dr nm where
W ( r inm
)
2
3 r 2 nm
3 / 2
4
2 r inm e
2
3 r
2 n m r
2 n m for the random-flight chain,
r nm
2
= | n m | l 2 . The average is obtained analytically sin( 2
Sr nm
2
Sr nm
)
e
4
2
S
2 r
2 nm
/ 6
The double sum over n and m can be converted to a single sum provided that only the difference | n m | matters. This seem reasonable as long as the distances of two segments of chain index by | n m | does not depend on the length of the chain. It is easy to envisage situations where this cannot be true, e.g. any part of a real chain will exclude its own
2
volume from the rest of the chain. However we are about to use distribution for the random flight chain that does not included the excluded volume effect either so the approximations are consistent with each other. Thus we obtain
I ( S )
4
f o
2
( S ) n
N
m
0
N
n
m
e
4
2
S
2 r
2 nm
/ 6
This has been solved by Debye (1947) to result in
I ( S )
2
e
x x 2 x
1
, x
4
2
S
2
R
G
2 where constants have been dropped for simplicity.
The result serves to be examined for the initial, central and final part of the scattering curve. Initially there is the
Gaussian region that allows us to extract the average size of the denatured ensemble. However the region is clearly shorter than for the folded species because other functionalities, seen by expanding the exponential term, come in the central region. Therefore the average size may not be so easy to determine in practise because the beam stopper will remove the primary beam, of course, but also the data at very small angles.
(Left I vs. S , Right S 2 I vs. S .)
The asymptotic behaviour at large S is of interest as well. Obviously the exponential part has by then decayed away and only remains
I ( S )
2 x x
2
1
2 x
1
1 x
, x
4
2 S 2 R 2
G
Apparently in the plot of S 2
I
vs. S approaches a constant value. Note that this 1/ S 2 dependence for coils is different form the 1/ S 4 dependence (Porod-law) mentioned earlier for folded proteins. Customarily a plot of S 2 I vs. S known as
Kratky-plot reveals the constant value that is characteristic to a coil.
For real polymers the asymptotic limit seems doubtful because no real chain element can be infinitely short. Thus we need to introduce a correction to the scattering by including scattering from a needle, i.e. the unbreakable unit element that we have earlier denoted by l
I ( S )
2
2
Sl
2
0
Sl sin( 2
Sl
2
Sl
)
2
Sdl
1
cos( 2
Sl )
2
Sl
Inspection of the asymptotic limit
I ( S )
2
2
Sl
2
1
2
Sl
2
Sl
4
2
2
S
2 l
2
3
reveals the 1/ S dependence. Ideally the intersection of the 1/ S 2 dependence that is a constant in the Kratky plot and the
1/ S dependence that is with a constant slope reveals the length of the chain segment. In practise this can be hard to determine accurately.
Note: a) Make sure you understand the form of scattering from folded and denatured proteins shown by Guinier or
Kratky plot or simply I vs S . b) Make sure you understand the derivation of the scattering from a molecule in the solution for the welldefined shapes and for the coil-like molecules.
4