Justification for the extrapolation of the experimental data to
advertisement

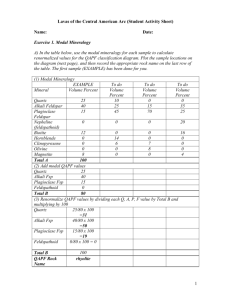
Supplementary Information 1 Justification for the extrapolation of the experimental data to high temperatures and pressures. To parameterize the partitioning of oxygen between magnesiowüstite and liquid iron, experimental data were used that were collected between 5 and 25 GPa and 2173-2673 K. In the model this parameterization is used to extrapolate the experimental observations up to 5000 K and 130 GPa. Although these extrapolations are large considering the range of experimental conditions, the conclusions of the model can be shown to be robust given the available constraints. The solubility of oxygen in Fe liquid increases with temperature, as can be seen clearly in Fig. 1c. This temperature trend is also supported by similar data of Li and Agee2 (Fig. 1c). The fit of Eq. 2 to our data is based on a constant value for the temperature dependence of KD, which we then use to extrapolate these data. One source of uncertainty in this extrapolation arises because ΔS in Eq. 2 is assumed to be a constant whereas in reality it will vary with temperature. In Fig. 1S, we show ΔS as a function of temperature calculated for a comparable reaction (the formation of pure iron oxide from the elements) for which thermodynamic data, and in particular heat capacity data, are available28,29. The agreement between the value calculated from thermodynamic data and the constant term determined by fitting Eq. 2 to our data is good considering that the calculated value is for a reaction involving the pure end member phases. What is more important, however, is that the ΔS term calculated from the thermodynamic data changes by less than 5 J/K between 2000 and 6000 K. This is consistent with the fact that the heat capacities of the phases involved, which can be extrapolated with good theoretical justification, change very little over this temperature range. We conclude, therefore, that the extrapolation of KD with temperature is robust and the uncertainties make no difference to the conclusions of our model. 80 Fe(l)+0.5O (g)=FeO(s) 2 75 70 S J/K 65 60 55 50 45 40 1000 2000 3000 4000 5000 6000 T/ K Figure S1. The temperature dependence of RTlnKD calculated from thermodynamic data for the reaction Fe(l)+0.5O2(g)=FeO(s) is shown by the dashed line. The temperature dependence of RTlnKD determined for our experimental data using Eq. 2 is shown by the solid line. The solubility of oxygen in liquid metal decreases with increasing pressure in the range 5-25 GPa as shown in Fig. 1b. As pointed out by O’Neill et al.15, this is consistent with the fact that the partial molar volume of FeO in liquid Fe measured at 1 bar (19 cm 3/mol at 1350°C) is much higher than the partial molar volume of FeO in magnesiowüstite (12.9 cm3/mol at the same temperature). The positive volume change of dissolving FeO in the metal therefore drives the solubility down with increasing pressure. In extrapolating the data, we ignore differences in the compressibilities of the two phases and therefore the dependence of the volume change of the reaction on pressure. In reality, given that the partial molar volume of FeO in liquid metal is high at 1 bar, its compressibility may also be significant. In this case, the volume change of dissolving FeO in liquid Fe could conceivably become positive at high pressure, which would reverse the pressure dependence of the oxygen solubility. However, oxygen solubilities decrease at least to 25 GPa and even if relative compressibilities do cause the sign of the reaction to change, a significant reversal of the oxygen solubility trend would only be expected to occur at pressures significantly above 25 GPa. The phase transformation observed by Fei and Mao30 raises the possibility of FeO becoming metallic at around 50 GPa; if this occurs, the miscibility gap between FeO and Fe may be significantly reduced and oxygen solubility may also rise. Therefore, at pressures significantly above 25 GPa, we cannot exclude the possibility that oxygen solubility may start to increase again either as a result of relative compressibilities or metallization of FeO. However, this makes no difference to our conclusions on the FeO contents of the mantles of Earth and Mars. As the Martian mantle only extends to pressures of about 25 GPa it will not be affected by a reversal in oxygen solubility in liquid Fe because this can only occur at significantly higher pressures. A reversal in oxygen solubility at higher pressures would affect the results for Earth and, in combination with relatively high temperatures, would reduce the depth of a magma ocean that is required to produce the present FeO content of the mantle. In this case, the FeO that was removed from the mantle by core formation now likely resides in the Earth’s core.