doc
advertisement
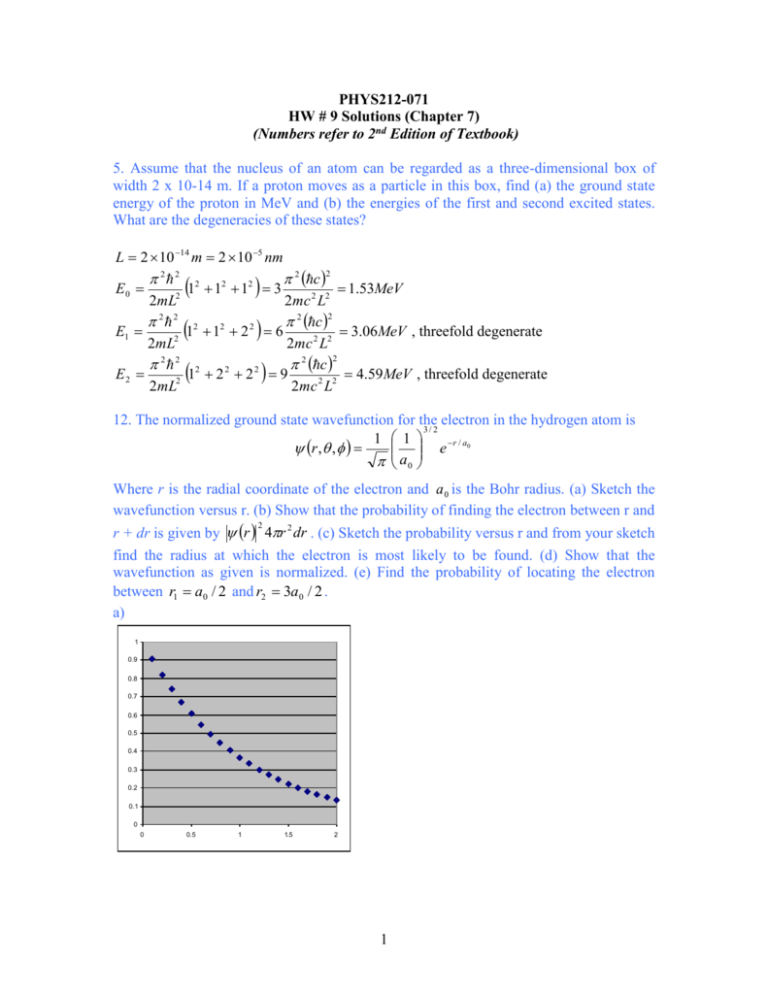
PHYS212-071 HW # 9 Solutions (Chapter 7) (Numbers refer to 2nd Edition of Textbook) 5. Assume that the nucleus of an atom can be regarded as a three-dimensional box of width 2 x 10-14 m. If a proton moves as a particle in this box, find (a) the ground state energy of the proton in MeV and (b) the energies of the first and second excited states. What are the degeneracies of these states? L 2 10 14 m 2 10 5 nm 2 2 2 2 2 2 c 2 E0 1 1 1 3 1.53MeV 2mL2 2mc 2 L2 2 2 2 2 2 c 2 2 E1 1 1 2 6 3.06MeV , threefold degenerate 2mL2 2mc 2 L2 2 2 2 2 c 2 2 2 E2 1 2 2 9 4.59 MeV , threefold degenerate 2mL2 2mc 2 L2 12. The normalized ground state wavefunction for the electron in the hydrogen atom is 3/ 2 1 1 e r / a0 r , , a0 Where r is the radial coordinate of the electron and a 0 is the Bohr radius. (a) Sketch the wavefunction versus r. (b) Show that the probability of finding the electron between r and 2 r + dr is given by r 4r 2 dr . (c) Sketch the probability versus r and from your sketch find the radius at which the electron is most likely to be found. (d) Show that the wavefunction as given is normalized. (e) Find the probability of locating the electron between r1 a0 / 2 and r2 3a0 / 2 . a) 1 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 0 0.5 1 1.5 2 1 2 0 0 3 b) d sin dd d d sin 4 r , , dV 2 1 1 a 0 2 r / a0 2 4 e r drd Pr 3 r 2 e 2 r / a0 a0 c) 0.16 0.14 0.12 0.1 0.08 0.06 0.04 0.02 0 0 0.5 1 1.5 2 2.5 3 3.5 The probability is maximum when r a 0 . d) drPr 0 e) p 4 4 1 r 2 e 2 r / a0 dr 3 a03 x 2 e 2 x dx 4 x 2 e 2 x dx 4 1 3 4 a0 0 a0 0 0 3 a0 / 2 3/ 2 Pr dr 4 x e 2 a0 / 2 2 x dx 4 0.124 0.5 1/ 2 19. A hydrogen atom is in the 6g state. (a) What is the principal quantum number? (b) What is the energy of the atom? (c) What are the values for the orbital quantum number and the magnitude of the electron’s orbital angular momentum? (d) What the possible values for the magnetic quantum number? For each value, find the corresponding z component of the electron’s orbital angular momentum and the angle that the orbital angular momentum vector makes with the z-axis. a) n 6 13.6eV 0.38eV 62 c) 4; 1 2 5 d) m 4,3,2,1,0,1,2,3,4 b) E cos m 1 m 2 5 deg rees 26.6,47.9,63.4,77.1,90,116.6,137.9,153.4,167.1 2 29. As shown in Example 7.9, the average distance of the electron from the proton in the hydrogen ground state is 1.5 bohrs. For this case calculate r , the uncertainty in the distance about the average value, and compare it with the average itself. Comment on the significance of your result. P1s 4 r 2 e 2 r / a0 3 a0 0 0 2 1/ 2 r rPr 4a0 x 3 e 2 x dx 4a0 r r 2 r r 2 r 2 Pr dr 0 r 3 3 a0 8 2 3 2 a0 4 r 3 a 0 0.866 a 0 2 2 3