Experimental_Section_Furans
advertisement

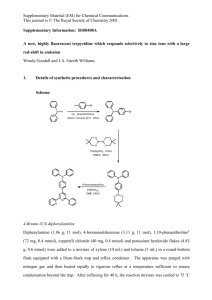
Experimental Section General methods: Chemicals (Aldrich, Fluka, Lancaster, and Merck) were used without further purification. Diethyl ether and tetrahydrofuran were dried over sodium. NMR spectra were recorded on Bruker ARX500, ARX300 and AMX250 spectrometers. Chemical shifts were referenced to residual solvent protons. Signal multiplicity as follows: s (singlet), d (doublet), t (triplet), achieved q via (quartet), DEPT90 m and (multiplet). DEPT135 13C spectra. assignment MS spectra was were recorded on a Finnigan MAT 90 a Varian 711 or a micrOTOF-Q spektrometer. IR spectra were recorded on a Bruker Vector 22. General procedure 1 (GP 1) – Addition of alkynes to α- chloroketones Under nitrogen, 1 eq. of the alkyne is dissolved in the specified solvent (THF or Et2O) and the solution is cooled to -78 °C. After slow addition of 1.05 eq. n-BuLi (1.6 M in hexane) the solution is stirred for 20 min. Finally 1 eq. of the chloroketone is added, the cooling bath is removed and the solution is stirred for 16 h at rt. After the addition of a saturated solution of NH4Cl, the phases are separated and the aqueous phase is extracted twice with DCM. The combined organic phases are dried over MgSO4, filtered and purified by column chromatography on silica (PE,EA). Dependent on the substrate, tertiary alcohols X, epoxides Y or both products are obtained. General procedure 2 (GP 2) – Conversion of tertiary alcohols X to epoxides Y: Under inert atmosphere 1 eq. of alcohol x is dissolved in dry Et2O (c = 0.4 mol/l). The solution is cooled to 0 °C and 2 eq. of freshly powdered NaOH are added in small portions. The reaction course is monitored by TLC. In some cases the solutions become highly viscous on addition of the base, if so the same amount of solvent should be added again. After complete conversion, the suspension is filtered, dried with MgSO4 and the solvent is removed. The raw products are purified by column chromatography (SiO2, PE, EA) General Procedure 3 (GP 3) - Gold catalysis 5 mol% gold catalyst X and 5 mol% AgOTf are stirred in a mixture of DCM/ MeOH (9/1) for 15 min. After the addition of the epoxide (c= 0.3 mol/l), the reaction mixture is stirred at rt and the reaction is monitored by TLC. After complete consumption of the starting material, the solvent is removed and the residue is purified by column chromatography on silica (PE, EA). Preparation of Verbindungsname Praktikant Nr Chem-Draw Struktur (Campus-Lizenz von Chemdraw verfügbar) According to GP 1 x.xx g (x.xx mmol) alkyne, x.xx ml Buli (1.6 M, in hexanes, x.xx mmol) and x.xx g (x.xx mmol) Name des Säurechlorids were converted in Solvent. After purification X.xx mg (x.xx mmol, x%) of the alcohol and x.xx mg (x.xx mmol, x%) of epoxide x were obtained as Farbe/ÖL Feststoff etc after purification by column chromatography (PE:EA, X:Y). ~ MP.: x°C; Rf (PE:EA, x:x) = x; IR (neat): = x cm-1, x; 1H NMR (Frequenz MHz, solvent): δ = x (Multiplizität, J = x.x Hz, x H), x; 13C NMR (Frequenz MHz, solvent): x (Multiplizität), ,; Masse: wird hier beim Abtippen der Daten näher erklärt: Preparation of Verbindungsname Praktikant Nr: Struktur According to GP 2 x.xx g (x.xx mmol) alcohol x and x.xx g (x.xx mmol) NaOH were chromatography (PE: EA, converted x:x), x.xx for x h. After g (x.xx mmol, column x%) were gained as Farbe/ÖL Feststoff etc. ~ MP.: x°C; Rf (PE:EA, x:x) = x; IR (neat): 1H = x cm-1, x,; NMR (Frequenz MHz, solvent): δ = x (Multiplizität, J = x.x Hz, x H), x; 13C NMR (Frequenz MHz, solvent): x (Multiplizität), ,; Masse: wird hier beim abtippen der Daten näher erklärt: Preparation of Verbindungsname Praktikant Nr: Struktur According to GP 3 x.xx mg (x.xx µmol) gold catalyst, x.xx mg (x.xx µmol) AgOTf and x.xx mg (x.xx mmol) epoxide X were converted for x h. After column chromatography (PE: EA, x:x), x.xx g (x.xx mmol, x%) were gained as Farbe/ÖL Feststoff etc. ~ MP.: x°C; Rf (PE:EA, x:x) = x; IR (neat): 1H = x cm-1, x,; NMR (Frequenz MHz, solvent): δ = x (Multiplizität, J = x.x Hz, x H), x; 13C NMR (Frequenz MHz, solvent): x (Multiplizität), ,; Masse: wird hier beim abtippen der Daten näher erklärt: