SC-82 Advanced Placement Physics
advertisement

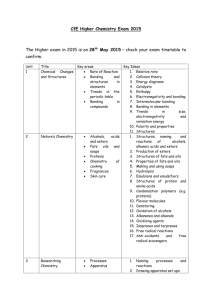
SC- Advanced Placement Chemistry Mesa High School Course Syllabus Course Title: Advanced Placement Chemistry Instructor: Elizabeth Fife Telephone: 480-472-5780 E-mail: ejfife@mpsaz.org Office: Mesa High School, room 41 Course Description: Advanced Placement Chemistry is an extremely rigorous, fast-paced, one-year course. The course of study includes stoichiometry, bonding and molecular geometry, the chemical properties of the major groups of elements, behavior of solids, liquids and gases, equilibrium theory, kinetics, thermodynamics, electrochemistry and other selected topics. The lab portion of the course is designed to give you the opportunity to manipulate equipment and materials to collect and analyze data and draw conclusions about the observed regularities. This process will either provide you an empirical basis for, or allow you to test your understanding of the concepts you will study in a given unit. Textbook: Chemistry Zumdahl, Steven S. and Susan A. (5th edition) – provided by Mesa High Prerequisites: Completion of a first year chemistry class is required. Concurrent enrollment or completion of trig or pre-calculus is recommended. A grade of “B” or higher in algebra II is recommended. Homework: Reading and homework problems will be assigned from the textbook and will be supplemented with practice AP test questions. Late work for each unit will be accepted for half credit up to the unit test; late work will not be accepted after the unit test. Lab AP Chemistry is a lab course. Students will be expected to keep a lab report portfolio as many colleges will wish to see evidence of lab work before giving course credit. Proper laboratory safety procedures will be observed during all lab work. On designated lab days, students are expected to spend a portion of the lunch period working on lab activities. (On average 1 hour every other week) Tests: Unit tests will cover the most current unit topics and will be scheduled as each unit is completed. Tests will not be graded on a curve and there will be no test retakes. Grading: Grades are cumulative throughout each semester; they will NOT start over at the quarter. Your grade will be determined by the following items: Tests and quizzes: 55% Lab reports: 25% Notebook (notes, class work, homework): 15% Practice free response questions (frq’s): 5% The grading scale will be: 88%-100% A 78-87% B 68-77% C 58-69% D 0-57% F Materials: spiral notebook – college ruled (for notes, class work, homework) scientific calculator (graphing calculators are allowed but not required)* USB flash drive* pens, #2 pencils, highlighters small bottle of glue and/or glue stick** scissors** * available for use in class but HIGHLY recommended that you have your own ** available for use in class Attendance: This class will follow district guidelines for attendance. Any student exceeding 9 absences may be audited in the course. (An appeal must be granted in order to receive credit.) Students must be on time to receive credit for warmups / bellwork. Discipline: All district guidelines presented in the student handbook and/or posted in the classroom will be followed at all times. Failure to comply with a reasonable request is considered Defiance and will be dealt with as indicated in the Student Behavior and Discipline Guidelines. Disclaimer: Course content and outline may vary from this syllabus to meet the needs of this group of students. Course Outline First Semester I. Chemical foundations (1.5-2 weeks) classification of matter measuring and significant figures dimensional analysis intro to atomic theory and structure inorganic nomenclature Lab – separation and analysis by chromatography (2 hours) 2. Intro to Stoichiometry (2.5 weeks) chemical mole balancing equations mass stioichiometry percent yield and limiting reagents determination of empirical and molecular formulas Lab – gravimetric analysis of a metal carbonate (2 hours) empirical formula of Cu?I? (2 hours) 3. Types of chemical reactions and solution stoichiometry (2.5 weeks) characteristics of electrolytes molarity calculations ionic equations for acids, bases, and salts stoich of precipitation reactions writing and balancing redox reactions Lab – redox titration (2 hours) separation and qualitative determination of cations and anions (3 hours) 4. Gases (2 weeks) properties of gases ideal gas law kinetic molecular theory Graham’s law van der Waal’s equation Lab – determination of the molar mass of gases and volatile liquids (2 hours) molar volume of a gas (2 hours) 5. Introduction to Energy, Thermodynamics, and Entropy (3.5 weeks) storage and transfer of energy enthalpy and PV work calorimetry Hess’s Law spontaneous processes and entropy Second law of thermodynamics Gibb’s free energy Lab – enthalpy of reaction and Hess’s Law 2 hours) 7. Atomic Structure and Periodicity (2 weeks) line spectra Bohr model quantum mechanics electron configuration ionization energy periodic trends Lab – analyzing spectra: flame tests / gas tubes (H and others) (1 hour) 8. Bonding and molecular architecture (3 weeks) chemical bonding – ionic and covalent lattice energy electronegativity and bond polarity average bond energies and ΔH VSPER, Lewis structures, and molecular orbital model resonance hybridization bonding in homonuclear and heteronuclear diatomic molecules Lab – VSPER lab – making molecular models (2 hours) Second Semester 9. Solids and Liquids (2 weeks) intromolecular forces interactions in liquids bonding and structures in metals and solids vapor pressure and changes of state phase diagrams 10. Solutions (2 weeks) solution composition energy and entropy in solution formation vapor pressure colligative properties colloids Lab – using freezing point depression to determine molar mass (2 hours) 11. Kinetics (2.5 weeks) reaction rates differential and integrated rate laws reaction mechanisms collision theory activation energy catalysts Lab – rate and order of a chemical reaction (2 hours) 12. Chemical Equilibrium (2 weeks) equilibrium condition equilibrium constant and rate constants for opposing reactions equilibrium expressions involving pressure and concentration LeChatelier’s principle free energy and equilibrium Lab – colorimetric determination of an equilibrium constant (3 hours) 13. Acids and bases (2 weeks) Arrhenius, Bronsted, and Lewis models of acids and bases pH scale effect of structure on acid-base properties acid-base properties of oxides 14. Applications of Aqueous Equilibria (2.5 weeks) common ion solutions buffered solutions titrations and pH curves solubility equilibria and Ksp equilibria and complex ions Lab – standardizing a solution and determination of the Ka of a weak acid (3 hours) pH properties of buffer solutions (3 hours) 15. Electrochemistry (2 weeks) oxidation-reduction reactions galvanic cells standard reduction and cell potentials electrical work and free energy Nernst equation batteries, corrosion, electrolysis Lab – constructing voltaic cells (1 hour) factors affecting electrochemical cells (2 hours) 16. Organic chemistry (1.5 weeks) structure and nomenclature of alkanes, alkenes, and alkynes structural isomerism aromatic hydrocarbons functional groups and derivatives Lab – synthesis, isolation, and purification of an ester (2 hours) Independent Study – Nuclear chemistry nuclear stability and radioactive decay kinetics of radioactive decay detection and uses of radioactivity radiocarbon dating