CHAPTER 25
advertisement

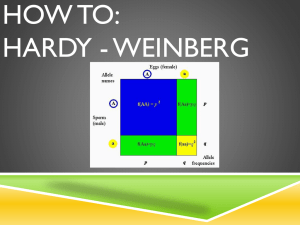
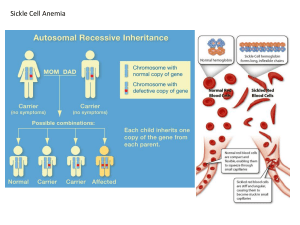
CHAPTER 25 Application and Experimental Questions E1. You will need to be familiar with the techniques described in Chapter 19 to answer this question. Gene polymorphisms can be detected using a variety of cellular and molecular techniques. Which techniques would you use to detect gene polymorphisms at the following levels? A. DNA level B. RNA level C. Polypeptide level Answer: A. At the DNA level, a clear-cut way to determine genetic variation is to clone and sequence genes. If the same gene is cloned from two different individuals and the sequences are different, this shows that there is genetic variation. In addition, several other methods can be used to detect genetic variation. For example, a comparison of Southern blots using samples from different individuals might reveal that a gene exists in different sizes or that it contains different restriction sites. B. At the RNA level, a Northern blot may reveal genetic variation. If the RNA encoded by two different alleles has a different size, this can be detected in a Northern blot. C. At the protein level, gel electrophoresis may reveal genetic variation. This method is described in the Appendix. Another approach is to study the function of an enzyme using a biochemical assay of its activity. E2. You will need to understand Solved problem S4 to answer this question. As described in Chapter 5, the gene for coat color in rabbits can exist in four alleles termed C (full coat color), c ch (chinchilla), c h (Himalayan), and c (albino). In a population of rabbits in Hardy-Weinberg equilibrium, the allele frequencies are C = 0.34 c ch = 0.17 c h = 0.44 c = 0.05 Assume that C is dominant to the other three alleles. cch is dominant to ch and c, and ch is dominant to c. A. What is the frequency of albino rabbits? B. Among 1,000 rabbits, how many would you expect to have a Himalayan coat color? C. Among 1,000 rabbits, how many would be heterozygotes with a chinchilla coat color? Answer: Solved problem S4 shows how the Hardy-Weinberg equation can be modified to include situations of three or more alleles. In this case: (p + q + r + s)2 = 1 p 2 + q 2 + r 2 + s 2 + 2pq + 2qr +2qs +2rp + 2rs + 2sp = 1 Let p = C, q = c ch, r = c h, and s = c. A. The frequency of albino rabbits is s 2: s2 = (0.05) = 0.0025 = 0.25% B. Himalayan is dominant to albino but recessive to full and chinchilla. Therefore, Himalayan rabbits would be represented by r 2 and by 2rs: r 2 + 2rs = (0.44)2 + 2(0.44)(0.05) = 0.24 = 24% Among 1,000 rabbits, about 240 would have a Himalayan coat color. C. Chinchilla is dominant to Himalayan and albino but recessive to full coat color. Therefore, heterozygotes with chinchilla coat color would be represented by 2qr and by 2qs. 2qr + 2qs = 2(0.17)(0.44) + 2(0.17)(0.05) = 0.17, or 17% Among 1,000 rabbits, about 170 would be heterozygotes with chinchilla fur. E3. In a large herd of 5,468 sheep, 76 animals have yellow fat, compared to the rest of the members of the herd, which have white fat. Yellow fat is inherited as a recessive trait. This herd is assumed to be in Hardy-Weinberg equilibrium. A. What are frequencies of the white and yellow fat alleles in this population? B. Approximately how many sheep with white fat are heterozygous carriers of the yellow allele? Answer: A. Let W represent the white fat allele and w represent the yellow fat allele. Assuming Hardy-Weinberg equilibrium, we can let p2 represent the genotype frequency of WW animals, and then Ww would be 2pq and ww would be q2. The only genotype frequency we know is that of the ww animals. ww q 2 76 5,468 q2 = 0.014 q = 0.12, which is the allele frequency of w p=1–q p = 0.88, which is the allele frequency of W B. The heterozygous carriers are represented by 2pq. If we use the values of p and q, which were calculated in part A: 2pq = 2(0.88)(012) = 0.21 Approximately 21% of the animals would be heterozygotes with white fat. If we multiply 0.21 times the total number of animals in the herd: 0.215,468 = 1,148 animals E4. The human MN blood group is determined by two codominant alleles, M and N. The following data were obtained from various human populations: Percentages Population Place MM MN NN Inuit East Greenland 83.5 15.6 0.9 Navajo Indians New Mexico 84.5 14.4 1.1 Finns Karajala 45.7 43.1 11.2 Russians Moscow 39.9 44.0 16.1 Aborigines Queensland 2.4 30.4 67.2 (Data from Speiss, E. B. (1990) Genes in Populations, 2d ed. NY:Wiley-Liss.) A. Calculate the allele frequencies in these five populations. B. Which populations appear to be in Hardy-Weinberg equilibrium? C. Which populations do you think have had significant intermixing due to migration? Answer: A. Eskimo M = 0.913 N = 0.087 Navajo M = 0.917 N = 0.083 Finns M = 0.673 N = 0.327 Russians M = 0.619 N = 0.381 Aborigines M = 0.176 N = 0.824 B. To determine if these populations are in equilibrium, we can use the Hardy-Weinberg equation and calculate the expected number of individuals with each genotype. For example: Eskimo MM = (0.913)2 = 83.3 MN = 2 (0.913)(0.087) = 15.9 NN = (0.087)2 = 0.76 In general, the values agree pretty well with an equilibrium. The same is true for the other four populations. C. Based on similar allele frequencies, the Eskimo and Navajo Indians seem to have interbred as well as the Finns and Russians. E5. You will need to understand Solved problem S4 before answering this question. In an island population, the following data were obtained regarding the numbers of people with each of the four blood types: Type O 721 Type A 932 Type B 235 Type AB 112 Is this population in Hardy-Weinberg equilibrium? Explain your answer. Answer: The first thing we need to do is to determine the allele frequencies. Let’s let p represent i, q represent IA, and r represent IB. p2 is the genotype frequency of ii q2 is the genotype frequency of IAIA r 2 is the genotype frequency of IBIB 2pq is the genotype frequency of IAi 2pr is the genotype frequency of IBi 2qr is the genotype frequency of IAIB p2 721 721 932 235 112 p2 = 0.36 p = 0.6 Next, we can calculate the allele frequency of IA. Keep in mind there are two genotypes (IAIA and IAi) that result in type A blood. q 2 2 pq 932 721 932 235 12 q2 + 2(0.6)q = 0.47 By solving this quadratic equation, we get q = 0.31 Now we can solve for r, p+q+r=1 0.6 + 0.31 + r = 1 r = 0.09 Based on these allele frequencies, we can compare the observed and expected values. To determine the expected values, we multiply the genotype frequencies times 2,000, which was the total number of individuals in this population. p2 is the genotype frequency of ii = (0.6)2(2,000) = 720 q2 is the genotype frequency of IAIA = (0.31)2(2,000) = 192 r 2 is the genotype frequency of IBIB = (0.09)2(2,000) = 16 2pq is the genotype frequency of IAi = 2(0.31)(0.6)(2,000) = 744 2pr is the genotype frequency of IBi = 2(0.09)(0.6)(2,000) = 216 2qr is the genotype frequency of IAIB = 2(0.31)(0.09)(2,000) = 111 Expected Numbers Observed Numbers Type O 720 721 Type A 192 + 744 = 936 932 Type B 16 + 216 = 232 235 Type AB 111 112 The observed and expected values agree quite well. Therefore, it does appear that this population is in Hardy-Weinberg equilibrium. E6. In a donor population, the allele frequencies for the normal (HbA) and sickle-cell alleles (HbS) are 0.9 and 0.1, respectively. A group of 550 individuals migrates to a new population containing 10,000 individuals; in the recipient population, the allele frequencies are HbA = 0.99 and HbS = 0.01. A. Calculate the allele frequencies in the conglomerate population. B. Assuming that the donor and recipient populations are each in HardyWeinberg equilibrium, calculate the genotype frequencies in the conglomerate population prior to further mating between the donor and recipient populations. C. What will be the genotype frequencies of the conglomerate population in the next generation, assuming that it achieves Hardy-Weinberg equilibrium in one generation? Answer: A. ∆pC = m(pD – pR) With regard to the sickle-cell allele: ∆pC = (550/10,550)(0.1 – 0.01) = 0.0047 pC = pR + ∆pC = 0.01 + 0.0047 = 0.0147 B. We need to calculate the genotypes separately: For the 550 migrating individuals, HbAHbA = (0.9)2 = 0.81, or 81% We expect (0.81)550 = 445.5 individuals to have this genotype HbAHbS = 2(0.9)(0.1) = 0.18 We expect (0.18)550 = 99 heterozygotes HbSHbS = (0.1)2 = 0.01 We expect (0.01)550 = 5.5 HbSHbS For the original recipient population, HbAHbA = (0.99)2 = 0.98 We expect 9,801 individuals to have this genotype HbAHbS = 2(0.99)(0.01) = 0.0198 We expect 198 with this genotype HbSHbS = (0.01)2 = 0.0001 We expect 1 with this genotype To calculate the overall population: (445.5 + 9801)/10,550 = 0.971 HbAHbA homozygotes (99 + 198)/10,550 = 0.028 heterozygotes (5.5 + 1)/10,550 = 0.00062 HbSHbS homozygotes C. After one round of mating, the allele frequencies in the conglomerate (calculated in part A), should yield the expected genotype frequencies according to the Hardy-Weinberg equilibrium. Allele frequency of HbS = 0.0147, so HbA = 0.985 HbAHbA = (0.985)2 = 0.97 HbAHbS = 2(0.985)(0.0147) = 0.029 HbSHbS = (0.0147)2 = 0.0002 E7. A recessive lethal allele has achieved a frequency of 0.22 due to genetic drift in a very small population. Based on natural selection, how would you expect the allele frequencies to change in the next three generations? Note: Your calculation can assume that genetic drift is not altering allele frequencies in either direction. Answer: Let’s assume that the relative fitness values are 1.0 for the dominant homozygote and the heterozygote and 0 for the recessive homozygote. The first thing we need to do is to calculate the mean fitness of the population. p 2WAA 2 pqWAa q 2Waa W (0.78) 2 2(0.78)(0.22) W W 0.95 The allele frequency (p) in the next generation equals p= p= q= p2WAA + pqWAa _ _ W W 0.82 1 - p = 0.18 We would follow the same general strategy for the second and third generations as well. For the second generation, the mean fitness of the population now equals 0.97. Using the preceding equation, the allele frequency of A in the second generation approximately equals 0.85. The frequency of the recessive allele in the second generation would equal about 0.15 and the mean fitness would now be approximately 0.98. The allele frequency of A in the third generation would be approximately 0.87. The frequency of the recessive allele would be about 0.13. E8. Among a large population of 2 million gray mosquitoes, one mosquito is heterozygous for a body color gene; this mosquito has one gray allele and one blue allele. There is no selective advantage or disadvantage between gray and blue body color. All of the other mosquitoes carry the gray allele. A. What is the probability of fixation of the blue allele? B. If fixation happens to occur, how many generations is it likely to take? C. Qualitatively, how would the answers to parts A and B be affected if the blue allele conferred a slight survival advantage? Answer: A. Probability of fixation = 1/2N (Assuming equal numbers of males and females contributing to the next generation) Probability of fixation = 1/2(2,000,000) = 1 in 4,000,000 chance B. _ t = 4N Where t = the average number of generations to achieve fixation N = the number of individuals in population, assuming that males and females contribute equally to each succeeding generation _ t = 4(2 million) = 8 million generations C. If the blue allele had a selective advantage, the value calculated in part A would be slightly larger; there would be a higher chance of allele fixation. The value calculated in part B would be smaller; it would take a shorter period of time to reach fixation. E9. Resistance to the poison warfarin is a genetically determined trait in rats. Homozygotes carrying the resistance allele (WW) have a lower fitness because they suffer from vitamin K deficiency, but heterozygotes (Ww) do not. However, the heterozygotes are still resistant to warfarin. In an area where warfarin is applied, the heterozygote has a survival advantage. Due to warfarin resistance, the heterozygote is also more fit than the normal homozygote (ww). If the relative fitness values for Ww, WW, and ww individuals are 1.0, 0.37, and 0.19 in areas where warfarin is applied, calculate the allele frequencies at equilibrium. How would this equilibrium be affected if the rats were no longer exposed to warfarin? Answer: The selection coefficients are sww = 1 – 0.19 = 0.81 sWW = 1 – 0.37 = 0.63 If the rats are not exposed to warfarin, the equilibrium will no longer exist, and natural selection will tend to eliminate the warfarin-resistance allele because the homozygotes are vitamin K deficient. E10. Describe, in as much experimental detail as possible, how you would test the hypothesis that snail color distribution is due to predation. Answer: You could mark snails with a dye and release equal numbers of dark and light snails into dimly lit forested regions and sunny fields. At a later time, recapture the snails and count them. It would be important to have a method of unbiased recapture because the experimenter would have an easier time locating the light snails in a forest and the dark snails in a field. Perhaps one could bait the region with something that the snails like to eat and only collect snails that are at the bait. In addition to this type of experiment, one could also observe predation as it occurs. E11. In the Grants’ study of the medium ground finch, do you think the pattern of natural selection was directional, stabilizing, disruptive, or balancing? Explain your answer. If the environment remained dry indefinitely (for many years), what do you think would be the long-term outcome? Answer: This is an example of directional selection. Over the long run, directional selection may lead to the loss of certain alleles and the fixation of others. In this case, alleles promoting smaller beak size might be lost from the population, while alleles promoting larger beak size could become fixed. E12. Here are traditional DNA fingerprints of five people: a child, mother, and three potential fathers: [Insert Text Art 25.7] Which males can be ruled out as being the father? Explain your answer. If one of the males could be the father, explain the general strategy for calculating the likelihood that he could match the offspring’s DNA fingerprint by chance alone. (See Solved problem S6 before answering this question.) Answer: Male 2 is the potential father, because he contains the bands that are found in the offspring but are not found in the mother. To calculate the probability, one would have to know the probability of having each of the types of bands that match. In this case, for example, male 2 and the offspring have four bands in common. As a simple calculation, we could eliminate the four bands that the offspring shares with the mother. If the probability of having each paternal band is 1/4, the chances that this person is not the father are (1/4)4. E13. What is DNA fingerprinting? How can it be used in human identification? Answer: DNA fingerprinting is a method of identification based on the properties of DNA. Minisatellites and microsatellite sequences are variable with regard to size in natural populations. This variation can be seen when DNA fragments are subjected to gel electrophoresis. Within a population, any two individuals (except for identical twins) will display a different pattern of DNA fragments, which is called their DNA fingerprint. E14. When analyzing the DNA fingerprints of a father and his biological daughter, a technician examined 50 bands and found that 30 of them were a perfect match. In other words, 30 out of 50 bands, or 60%, were a perfect match. Is this percentage too high, or would you expect a value of only 50%? Explain why or why not. Answer: This percentage is not too high. Based on their genetic relationship, we expect that a father and daughter must share at least 50% of the same bands in a DNA fingerprint. However, the value can be higher than that because the mother and father may have some bands in common, even though they are not genetically related. For example, at one site in the genome, the father may be heterozygous for a 4,100 bp and 5,200 bp minisatellite, and the mother may also be heterozygous in this same region and have 4,100 bp and 4,700 bp minisatellites. The father could pass the 5,200 bp band to his daughter, and the mother could pass the 4,100 bp band. Thus, the daughter would inherit the 4,100 bp and 5,200 bp bands. This would be a perfect match to both of the father’s bands, even though the father transmitted only the 5,200 bp band to his daughter. The 4,100 bp band matches because the father and mother happened to have a minisatellite in common. Therefore, the 50% estimate of matching bands in a DNA fingerprint based on genetic relationships is a minimum estimate. The value can be higher than that. E15. What would you expect to be the minimum percentage of matching bands in a DNA fingerprint for the following pairs of individuals? A. Mother and son B. Sister and brother C. Uncle and niece D. Grandfather and grandson Answer: The minimum percentage of matching bands is based on the genetic relationships. A. 50% B. 50% (on average, but it could be less or more) C. 25% (on average, but it could be less or more) D. 25% (on average, but it could be less or more)