הודעה על החמרה ( מידע בטיחות) בעלון לרופא
advertisement
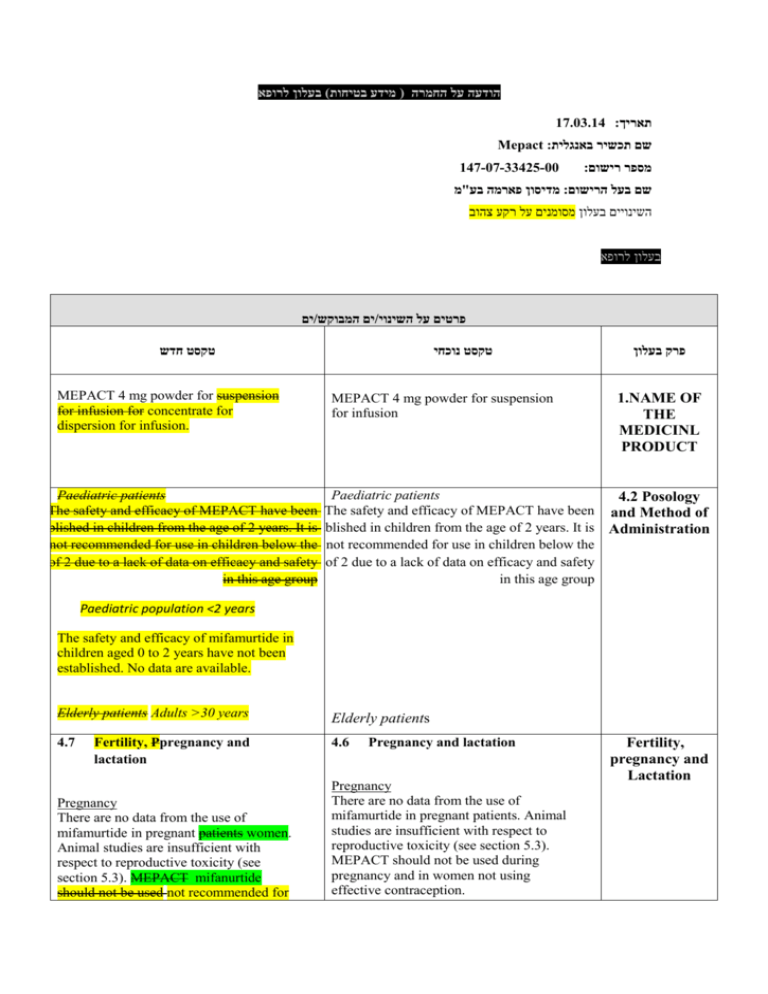
הודעה על החמרה ( מידע בטיחות) בעלון לרופא 17.03.14 :תאריך Mepact :שם תכשיר באנגלית 147-07-33425-00 :מספר רישום מדיסון פארמה בע"מ:שם בעל הרישום השינויים בעלון מסומנים על רקע צהוב בעלון לרופא ים/ים המבוקש/פרטים על השינוי טקסט חדש MEPACT 4 mg powder for suspension for infusion for concentrate for dispersion for infusion. טקסט נוכחי MEPACT 4 mg powder for suspension for infusion פרק בעלון 1.NAME OF THE MEDICINL PRODUCT Paediatric patients Paediatric patients 4.2 Posology The safety and efficacy of MEPACT have been The safety and efficacy of MEPACT have been and Method of established in children from the age of 2 years. Itestablished is in children from the age of 2 years. It is Administration not recommended for use in children below the not recommended for use in children below the age of 2 due to a lack of data on efficacy and safety age of 2 due to a lack of data on efficacy and safety in this age group in this age group Paediatric population <2 years The safety and efficacy of mifamurtide in children aged 0 to 2 years have not been established. No data are available. Elderly patients Adults >30 years Elderly patients 4.7 4.6 Fertility, Ppregnancy and lactation Pregnancy There are no data from the use of mifamurtide in pregnant patients women. Animal studies are insufficient with respect to reproductive toxicity (see section 5.3). MEPACT mifanurtide should not be used not recommended for Pregnancy and lactation Pregnancy There are no data from the use of mifamurtide in pregnant patients. Animal studies are insufficient with respect to reproductive toxicity (see section 5.3). MEPACT should not be used during pregnancy and in women not using effective contraception. Fertility, pregnancy and Lactation use during pregnancy and in women of childbearing potential not using effective contraception. Lactation Breast-feeding It is unknown whether mifamurtide is excreted in human milk. The excretion of mifamurtide in milk has not been studied in animals. A decision on whether to continue/discontinue breast-feeding or to continue/discontinue therapy should be made taking into account the benefit of breast-feeding to the child and the benefit of MEPACT mifanurtide therapy to the woman. Fertility No dedicated fertility studies have been conducted with mifamurtide (see section 5.3). Lactation It is unknown whether mifamurtide is excreted in human milk. The excretion of mifamurtide in milk has not been studied in animals. A decision on whether to continue/discontinue breast-feeding or to continue/discontinue therapy should be made taking into account the benefit of breast-feeding to the child and the benefit of MEPACT therapy to the woman. Undesirable effects Summary of the safety profile Mifamurtide was studied as a single agent in Each of the 248 patients treated with mostly advanced malignancies MEPACT during the early single arm phase single arm I and II clinical studies in patients with mostly advanced malignancies experienced at least one undesirable effect. The most frequent adverse reactions, occurring in >50% of patients, were chills, pyrexia, fatigue, nausea, tachychardia and headache. Many of the most frequently very commonly reported undesirable effects adverse reactions as shown in the following summary table are thought to be related to the mechanism of action of mifamurtide (see Table 1). The majority of these events were reported as either mild or moderate. This profile is consistent whether summarising all early studies (n=248) or only those studies in osteosarcoma (n=51). It is likely that these undesirable effects adverse reactions also occurred in the large randomised study, but they were not recorded because only serious and life-threatening adverse reactions were collected in that study. Undesirable effects Each of the 248 patients treated with MEPACT during the early phase single arm studies in patients with mostly advanced malignancies experienced at least one undesirable effect. Many of the most frequently reported undesirable effects as shown in the following summary table are thought to be related to the mechanism of action of mifamurtide. The majority of these events were reported as either mild or moderate. This profile is consistent whether summarising all early studies (n=248) or only those studies in osteosarcoma (n=51). It is likely that undesirable effects also occurred in the large randomised study, but they were not recorded because only serious and life-threatening adverse reactions were collected in that study. Blood and lymphatic system disorders Anaemia has most very commonly been reported when MEPACT mifanurtide is used in conjunction with chemotherapeutic agents. In a randomised controlled trial study, the incidence of myeloid malignancy (acute myeloid leukaemia/myelodysplastic syndrome) was the same in patients receiving MEPACT plus chemotherapy as in patients receiving only chemotherapy (approximately 2.15%). Blood and lymphatic system disorders Anaemia has most commonly been reported when MEPACT is used in conjunction with chemotherapeutic agents. In a randomised controlled trial, the incidence of myeloid malignancy (acute myeloid leukaemia/myelodysplastic syndrome) was the same in patients receiving MEPACT plus chemotherapy as in patients receiving only chemotherapy (approximately 2.5%). Metabolism and nutritional disorders Anorexia (21%) was very commonly reported in trials of MEPACT in late stage cancer patients phase I and II Metabolism and nutritional disorders Anorexia (21%) was very commonly reported in trials of MEPACT in late stage cancer patients. Undesirable effects studies of mifamurtide. Nervous system disorders Consistent with other generalised symptoms, the most common nervous system disorders were headache (50%) and dizziness (17%). One patient in the phase III study experienced 2 episodes of Grade 4 seizure while on study therapy with chemotherapy and mifamurtide. The second episode involved multiple grand mal seizures over the course of days. Mifamurtide treatment was continued for the remainder of the study without seizure recurrence. Nervous system disorders Consistent with other generalised symptoms, the most common nervous system disorders were headache (50%) and dizziness (17%). Cardiac and vascular disorders Cardiac and vascular disorders Mild-moderate tachycardia (50%), hypertension Mild-moderate tachycardia (50%), (26%) and hypotension (29%) were commonly hypertension (26%) and hypotension (29%) were very commonly reported in reported in uncontrolled trials of MEPACT. uncontrolled trials studies of MEPACT mifanurtide. One serious incident of subacute thrombosis was reported in early studies, but no serious cardiac events were associated with MEPACT mifanurtide in a large randomised controlled trial study. Musculoskeletal and connective tissue disorders Low grade pain was very common in patients receiving MEPACT mifanurtide, including myalgia (31%), back pain (15%), extremity pain (12%) and arthralgia (10%). Immune system disorders In a phase I study, there was one report of severe allergic reaction occurring after the first infusion of mifamurtide at 6 mg/m2 dose level. The patient experienced shaking, chills, fever, nausea, vomiting, uncontrollable coughing, shortness of breath, cyanotic lips, dizziness, weakness, hypotension, tachycardia, hypertension and hypothermia leading to study discontinuation. There was also one report of a grade 4 allergic reaction (hypertension) requiring hospitalization in the phase III study (see section 4.4). Musculoskeletal and connective tissue disorders Low grade pain was common in patients receiving MEPACT, including myalgia (31%), back pain (15%), extremity pain (12%) and arthralgia (10%). לא קיים לא קיים Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions. 5.1 Pharmacodynamic properties Pharmacotherapeutic group: Immunostimulants, Other immunostimulants, Other cytokines and immunomodulators, ATC code: L03AX15 Nature and contents of container 5.1 Pharmacodynamic properties Pharmacodyna mic properties Pharmacotherapeutic group: Other cytokines and immunomodulators, ATC code: L03AX15 Nature and contents of container 50 ml type I glass vial with a grey butyl rubber stopper, aluminium seal and plastic flip-off cap, containing 4 mg of mifamurtide. 50 ml type I glass vial with a grey butyl rubber stopper, aluminium seal and plastic flip-off cap, containing 4 mg of mifamurtide. Each carton contains one vial and one single-use, non-pyrogenic, latex-free sterile Ffilter for MEPACT supplied in a PVC-grade blister. Each carton contains one vial and one single-use, non-pyrogenic, latex-free sterile Filter for MEPACT supplied in a PVC-grade blister. Nature and contents of container . שבו מסומנות ההחמרות המבוקשות על רקע צהוב,מצ"ב העלון יש לסמן רק תוכן מהותי ולא שינויים.שינויים שאינם בגדר החמרות סומנו (בעלון) בצבע על רקע ירוק .במיקום הטקסט









