Course Outline - Suffolk County Community College
advertisement

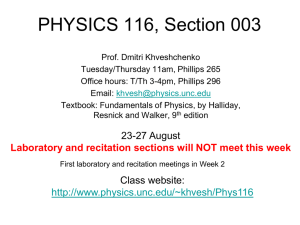
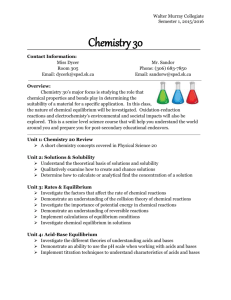
CH 33 1 SUFFOLK COUNTY COMMUNITY COLLEGE DEPARTMENT OF PHYSICAL SCIENCE CH33 Course Outline and Course Policy Lecture and Lab College Chemistry I: CH 33; Section 5142 Rm T-109 Mon, Tue, Wed & Thu - 6:00 - 07:15 PM (lectures) Mon & Wed - 7:30 - 8:20 PM (recitations) Tue & Thu - 07:30 PM-10:15 PM (lab; Rm T119) Summer 2008 Vishwas Joshi joshiv@sunysuffolk.edu www2.sunysuffolk.edu/joshiv password: ch33-sccc OBJECTIVES OF THE COURSE: This course is intended for the student whose emphasis is science, engineering, medicine, dentistry, chiropractic, physical therapy, veterinary science and art restoration. The successful student will be able to demonstrate a proficiency in: 1. Writing the name and formula for inorganic ionic and covalent compounds. 2. Describing the electronic structure of atoms and ions using the wave-mechanical theory. Perform simple calculations based on the wave-mechanical theory. 3. Performing computations involving stoichiometry under aqueous and non-aqueous conditions including the gaseous state and thermochemical situations. 4. Using V.S.E.P.R., M.O. and V.B. theories to describe complex molecules and ions; and deriving information regarding stability and properties. 5. Performing basic laboratory operations involving: gravimetric analysis and synthesis, visible spectroscopy, visible spectrophotometry, titration, boiling point and vapor density measurements, calorimetry and using molecular modeling materials. PREREQUISITE: MA 61 or permission of the Department Head and one year high school chemistry, CH19 or CH-29. PROCEDURES FOR ACCOMPLISHING THESE OBJECTIVES: Chemistry 33 consists of four one-hour and fifteen-minute lectures, two fifty-minute recitation and two two-hour and fifty-minute lab per week. The lecture periods will consist of formal lectures; recitation periods will be devoted to class discussion and problem solving. The laboratory period will begin with a short lecture on the current experiment. In the laboratory all work will be INDIVIDUAL unless the instructor indicates otherwise. No exposed feet (NO bare feet, open-toed shoes, or sandals) or bare midriffs will be allowed in the laboratory. CHEMISTRY DEPARTMENT APPROVED SAFETY GOGGLES MUST BE WORN AT ALL TIMES. If a student is 10 minutes late to lab and therefore misses the pre-lab presentation given by the instructor which includes safety precautions, the student will not be allowed to work in lab and this will result in a grade of zero for that lab. CH 33 2 STUDENT REQUIREMENTS FOR COMPLETION OF THE COURSE: a. You are expected to read the chapter assignments in the textbook and to complete any other assignments, which your instructor may require you to perform. b. You will be required to complete satisfactorily all lecture exams as well as a one-hour and fifteenminute final exam. No grades will be dropped and no one will be exempted from the final. c. The laboratory grade will be based on the pre-lab assignments and lab reports and technique. Laboratory reports are due the lab period following completion of the experiment. If you fail to submit a lab report you will receive a failing grade for that lab activity. Pre-lab assignments cannot be turned in late. Late lab reports will be penalized 5 points per school day. After being one week late, the report will not be accepted. d. Grading Practices: Your final grade will be determined as follows: Lecture exams, homework, quizzes 60% Final exam 15% Sub Total 75% Laboratory 25% Total 100% You must have passing grades in both lecture and laboratory to pass the course. You may withdraw from the course up to mid-term and receive a "W". If you withdraw after mid-term, you will receive a "W" if you are passing at the time of withdrawal and an "F" if you are failing. e. Rules Concerning Student Attendance: The college expects all students to attend every class session for which they are registered. Students are responsible for all that transpires in class whether or not they are in attendance. The College defines excessive absence or lateness as more than the equivalent of one week of class meetings during the semester. Excessive absence or lateness may lead to failure in a course or removal from the class roster. Examination Absence: Your instructor will provide you in writing with her/his policies for handling absences from examination. Laboratory Absence: There are NO make-up sessions for absence from laboratory. Your instructor will provide you in writing with her/his policies for handling absences from laboratory. f. Materials (available at SCC Bookstore): Required: check-in) 1. CH 33 Course textbook: Chemistry, 7th Ed., by Zumdahl/Zumdahl, 2007 Houghton Mifflin Company 2. Eduspace, Smarthinking, Active Learning Guide package (sold with textbook or may be purchased separately at Houghton Mifflin's website: www.college.hmo.com/students ) Instructor Code: VISHW-3D13ADFEF5575C 3. CH 33 Laboratory Manual: SCCC CH 33 Laboratory Manual (CER/Thomson) 4. IUPAC approved SAFETY GOGGLES (required for all lab sessions, including CH 33 3 WEEKLY OUTLINE OF LECTURE TOPICS Week Lecture Topic 1 1,2 3 4 4,5 5 6 6,7 7 8 8 Chemistry: Matter and Measurement Atoms, Molecules, and Ions Stoichiometry: Chemical Calculations Chemical Reactions in Aqueous Solutions Gases Thermochemistry Atomic Structure & Periodicity Bonding: General Concepts Covalent Bonding: Orbitals Liquids and Solids Review Textbook Chapter Reference 1 2 3 4 5 6 7 8 9 10 1-10 Chemistry 33 Course Outline Supplement: Course Policy for Dr. Vishwas Joshi Preparation: Homework is not optional. The assigned problems reflect the classroom material and an answer sheet will be given out in recitation on the date suggested for completion of each assignment. Homework assignments will be reviewed during recitation. Quizzes: Nine (9) Quizzes will be given in recitation or take home on the dates listed on the assignment sheet. The sum total of the quiz grades will count 10% towards your final course grade. Each quiz is worth 10 points for a total of 80 points (drop 1 lowest quiz). The quizzes will be 25 minutes (maximum) in length. There will be no makeup quizzes. Exams: Three exams will be given during class time on the dates listed and will be returned and reviewed during recitation. There will be NO makeup exams and no grade will be dropped. I may be reached at my cell-phone 455-3526 or joshiv@sunysuffolk.edu in the event of an emergency prior to a scheduled exam. Attendance: The college policy states that two absences (the equivalent of one week of class meeting time) constitutes excessive absence of course content for the student and are grounds for being dismissed from the course. If you are absent, you are responsible for any and all materials, notes, and class content (quizzes, answer sheets, handouts, prelabs, lab reports) that transpired during, or was due to be submitted in your absence. Cell Phones: Audible cell phone usage such as ringing during class, answering the phone during class, or text messaging during class is disruptive for everyone. While I realize that an emergency may arise that would necessitate your receiving a call, I require that all cell pones be placed in a vibrate mode and if you must take a call (for emergency only) that you remove yourself from the classroom/lab/recitation area before you answer in an audible manner. Calculators: A scientific calculator is required for the large amount of calculations that we will be performing in all aspects of this chemistry course. This does not have to be a state-of-the-art, top-of-theline model. A TI 13 X is sufficient, as long as you are able to perform scientific notation, logarithms, and that the calculator has some memory storage capacity. 1. YOUR CELL PHONE CANNOT SERVE AS A CALCULATOR. 2. CELL PHONES MAY NOT BE VISIBLE DURING QUIZZES OR EXAMINATIONS. CALCULATORS MAY NOT BE SHARED ON QUIZZES OR EXAMINATIONS CH 33 4 CHEMISTRY 33 LABORATORY Summer 2008 SCHEDULE CHEMISTRY 33 LABORATORY SCHEDULE Section 5142, Wednesday; RM T119; 07:30 PM-10:15 PM Lab Session Experiment Title Experiment Designation 06/03/2008 Laboratory Technique Check into Laboratory TECH 382 06/05/2008 Physical Properties: Determination of Density PROP 440 06/10/2008 Calculating Avogadro’s Number by Comparing Atoms and Oranges FORMAL LAB REPORT STOI 514 06/12/2008 Determining the Empirical Formula of a Compound STOI-423 06/17/2008 Single Replacement Reactions and Relative Reactivity Double Replacement Reactions REAC-389 REAC-390 06/19/2008 Separating and Determining the Mass of Calcium Ion in a Calcium-Enriched Tablet ANAL-455 06/24/2008 The Titration of Acids and Bases Volumetric Analysis Handout 06/26/2008 Determination of the Molecular Volume of a Gas FORMAL LAB REPORT Handout 07/01/2008 Evaluation of 0º K PROP-362 07/03/2008 Enthalpy of Formation of Magnesium Oxide Handout 07/08/2008 Spectrophotometric Analysis of colored dye ANAL-359 07/10/2008 Visible Atomic Spectrum of Hydrogen STRC-345 07/15/2008 07/17/2008 Geometric Structure of Molecules, Check out STRC 409 All labs are found in the laboratory manual package purchased at the bookstore Bold face-Italicized type labs will be handed in as formal lab reports * CH 33 Laboratory Manual is available at the Bookstore. THIS COURSE OUTLINE REPRESENTS BASIC DEPARTMENTAL POLICY FOR ALL SECTIONS OF THIS COURSE. AN INDIVIDUAL INSTRUCTOR MAY AUGMENT THIS POLICY TO MEET THE PARTICULAR NEEDS OF THE CLASS. CH 33 5 CHEM 33 (S - 5142) V. Joshi Summer, 2008 Homework, Quiz, and Exam (Tentative) Schedule CHAPTER 1 2 3 4 5 6 7 8 9 10 END OF CHAPTER PROBLEMS 2,4,6,8,16,24,26,30,32,34,38,42,50,58,60,70,72 18,30,39,40,42,44,46,50,52,53,55,56,59,60,62,64,68,70 12,15,18,22,28,30,36,40,42,44,46,48,51,56,60,62,67,68,70, 74,82,84,86,90,92,98,104,109,110 3,10,12,14,18,20,21,24,26,30,36,38,44,46,53,56,60,64,70,72 7,11,18,20,28,34,36,38,40,52,53,61,62,66,70,76,79,81,84,85 32,34,35,38,42,45,47,58,61,62,67,73 3,32,33,36,40,46,50,58,60,63,66,69,74,83,86,88,90,94,98,104,106 3,6,12,24,28,29,34,36,40,42,54,56,57,67,68,71,75,81,82,94,98,99,103 6,10,14,16,18,27,28,33,36,40 10,12,20,30,32,34,36,91 EXAM I II III Final Exam CHAPTERS 1,2,3 4,5,6 7,8,9 1-10 cumulative DATE 06/16/2008 07/03/2008 07/17/2008 07/22/2008 Tentative Quiz Schedule (Most of them at Recitation) Location: Room T-109 7:30 - 8:20 PM Quiz 1 2 3 4 5 6 7 8 9 Chapters 1 2 3 (part) 3 (rest) 4 5 6 7 8 9 Date Take home 6/3/08, due 6/5/08 6/10/08 6/12/08 6/24/08 6/26/08 7/1/08 7/10/08 7/15/08 Take home 7/17; due 7/22/08 DUE DATE 6/4/08 6/10/08 6/12/08 6/23/08 6/26/08 7/2/08 7/8/08 7/14/08 7/16/08 7/21/08 CH 33 6 Chemistry 33 Course outline supplement: Vishwas joshi Laboratory Policy Preparation: All of the experiments include pre-lab assignments. For each of these experiments you must complete the pre laboratory assignment before coming to the laboratory session. This assignment is due at the beginning (PRE-LAB) of each laboratory period and counts as 10 points toward the grade for each experiment. Pre-lab assignments will not be accepted after the laboratory for which the assignment is due is in session. Attendance: Being on time for laboratory is essential. If you are late (more than 10 minutes) you are not allowed to carry out the experiment for that day and this will be counted as an absence. Laboratory absences cannot be made up. Grading: Laboratory reports are due one week after the experiment is completed. Late lab reports will be penalized five (5) points per school day for a maximum of 5 school days (or one school week). After one week, then report will not be accepted and will result in a grade of 0 (zero). One of the experiments requires two consecutive weeks to finish and therefore the grade will be counted twice. For this experiment, you must be present for both weeks to obtain the doubled lab grade. An unexcused laboratory absence will result in a grade of 0 (zero) for that experiment. The two lowest lab grades will be dropped if you are present for all of the experiments. If you miss a third lab, you will be dropped from the course. Lab grades are not separable or transferable from previous semesters or to subsequent semesters.