Abbott Laboratories
advertisement
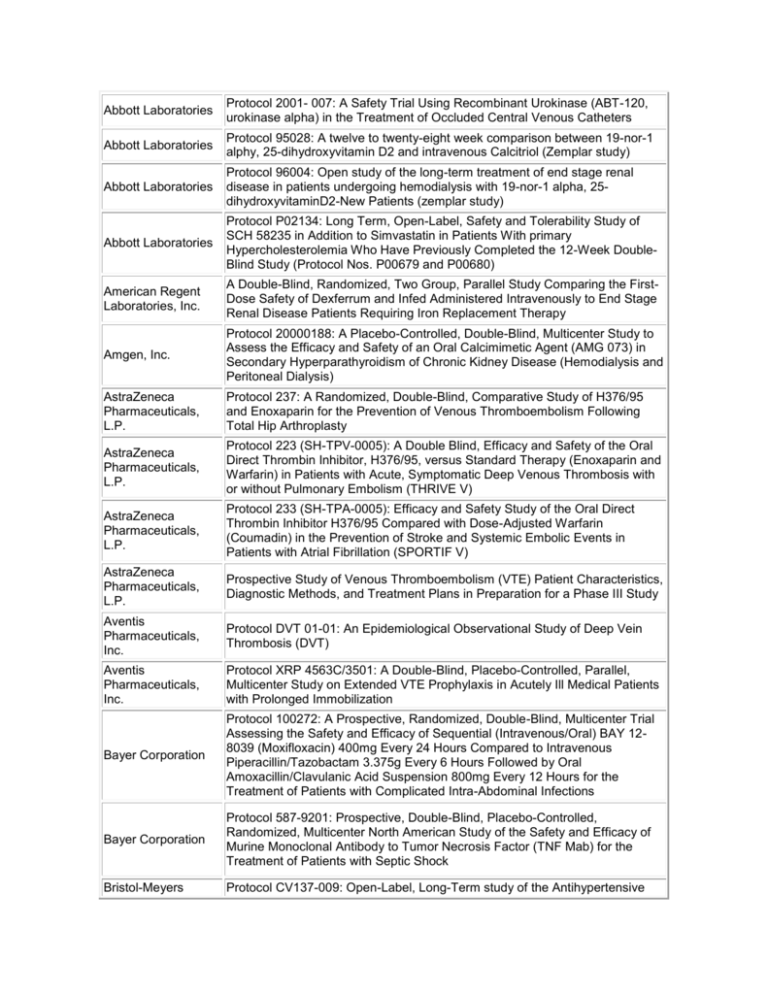
Abbott Laboratories Protocol 2001- 007: A Safety Trial Using Recombinant Urokinase (ABT-120, urokinase alpha) in the Treatment of Occluded Central Venous Catheters Abbott Laboratories Protocol 95028: A twelve to twenty-eight week comparison between 19-nor-1 alphy, 25-dihydroxyvitamin D2 and intravenous Calcitriol (Zemplar study) Abbott Laboratories Protocol 96004: Open study of the long-term treatment of end stage renal disease in patients undergoing hemodialysis with 19-nor-1 alpha, 25dihydroxyvitaminD2-New Patients (zemplar study) Abbott Laboratories Protocol P02134: Long Term, Open-Label, Safety and Tolerability Study of SCH 58235 in Addition to Simvastatin in Patients With primary Hypercholesterolemia Who Have Previously Completed the 12-Week DoubleBlind Study (Protocol Nos. P00679 and P00680) American Regent Laboratories, Inc. A Double-Blind, Randomized, Two Group, Parallel Study Comparing the FirstDose Safety of Dexferrum and Infed Administered Intravenously to End Stage Renal Disease Patients Requiring Iron Replacement Therapy Amgen, Inc. Protocol 20000188: A Placebo-Controlled, Double-Blind, Multicenter Study to Assess the Efficacy and Safety of an Oral Calcimimetic Agent (AMG 073) in Secondary Hyperparathyroidism of Chronic Kidney Disease (Hemodialysis and Peritoneal Dialysis) AstraZeneca Pharmaceuticals, L.P. Protocol 237: A Randomized, Double-Blind, Comparative Study of H376/95 and Enoxaparin for the Prevention of Venous Thromboembolism Following Total Hip Arthroplasty AstraZeneca Pharmaceuticals, L.P. Protocol 223 (SH-TPV-0005): A Double Blind, Efficacy and Safety of the Oral Direct Thrombin Inhibitor, H376/95, versus Standard Therapy (Enoxaparin and Warfarin) in Patients with Acute, Symptomatic Deep Venous Thrombosis with or without Pulmonary Embolism (THRIVE V) AstraZeneca Pharmaceuticals, L.P. Protocol 233 (SH-TPA-0005): Efficacy and Safety Study of the Oral Direct Thrombin Inhibitor H376/95 Compared with Dose-Adjusted Warfarin (Coumadin) in the Prevention of Stroke and Systemic Embolic Events in Patients with Atrial Fibrillation (SPORTIF V) AstraZeneca Pharmaceuticals, L.P. Prospective Study of Venous Thromboembolism (VTE) Patient Characteristics, Diagnostic Methods, and Treatment Plans in Preparation for a Phase III Study Aventis Pharmaceuticals, Inc. Protocol DVT 01-01: An Epidemiological Observational Study of Deep Vein Thrombosis (DVT) Aventis Pharmaceuticals, Inc. Protocol XRP 4563C/3501: A Double-Blind, Placebo-Controlled, Parallel, Multicenter Study on Extended VTE Prophylaxis in Acutely Ill Medical Patients with Prolonged Immobilization Bayer Corporation Protocol 100272: A Prospective, Randomized, Double-Blind, Multicenter Trial Assessing the Safety and Efficacy of Sequential (Intravenous/Oral) BAY 128039 (Moxifloxacin) 400mg Every 24 Hours Compared to Intravenous Piperacillin/Tazobactam 3.375g Every 6 Hours Followed by Oral Amoxacillin/Clavulanic Acid Suspension 800mg Every 12 Hours for the Treatment of Patients with Complicated Intra-Abdominal Infections Bayer Corporation Protocol 587-9201: Prospective, Double-Blind, Placebo-Controlled, Randomized, Multicenter North American Study of the Safety and Efficacy of Murine Monoclonal Antibody to Tumor Necrosis Factor (TNF Mab) for the Treatment of Patients with Septic Shock Bristol-Meyers Protocol CV137-009: Open-Label, Long-Term study of the Antihypertensive Squibb Activity and Safety of BMS-186716, A Dual Metalloprotease Inhibitor, in the Treatment of Hypertension Bristol-Meyers Squibb Protocol CV137-120: Omapatrilat Cardiovascular Treatment Assessment Versus Enalapril (OCTAVE) DuPont Pharmaceuticals Company Protocol DMP 702-401: A Phase IV, Multicenter, Prospective, Open-Label, Observational Study to Evaluate Clinical Practice Patterns of Innohep (Tinxaparin sodium injection) in the Treatment of Acute Symptomatic Deep Venous Thrombosis Eli Lilly and Company Protocol H6W-MC-MCAA: A Phase 2 Study to Determine the Efficacy and Safety of Sivelestat in Subjects with Acute Lung Injury Eli Lilly and Company Protocol H4Q-MC-ARRD: LY333328 versus Vancomycin in Skin/SkinStructure Infections Eli Lilly and Company Protocol F1D-US-HGJU: A Controlled Trial of Olanzapine versus Ziprasidone in the Treatment of Schizophrenic and Schizoaffective Subjects with Comorbid Depression Eli Lilly Research Laboratories Protocol F1K-MC-EVAD: A Phase III Study to Determine the Efficacy and Safety for Recombinant Human-Activated Protein C in Severe Sepsis Endo Pharmaceuticals Protocol EN3202.021: An Open-Label Extension Study to Evaluate the LongTerm Safety, Tolerability and Analgesic Efficacy of Numorphan CR (oxymorphone HCl controlled release) in Subjects with Cancer Pain or Chronic Lower Back Pain Endo Pharmaceuticals Protocol EN3202.019: A Randomized, Double-Blind, Two-Period Crossover Study Comparing the Efficacy, Safety and Tolerability of Numorphan CR (Oxymorphone HCl, Controlled Release) and OxyContin (Oxycodone HCl, Controlled release) in Cancer Patients who Require Chronic Opioid Treatments GlaxoSmithKline Protocol SB265805/287: An Open Label, Non-Comparative Study to Assess the Efficacy and Safety of Oral Gemifloxacin 320mg, Once Daily for Seven Days, for the Treatment of Community-Aquired Pneumonia of Suspected Pneumoncoccal Origin in Countries with a High Prevalence of Drug-Resistant Respiratory Pathogens GlaxoSmithKline Protocol 208127/091: A phase 4, open, randomized, controlled, comparative, multicenter U.S. study of the safety of GlaxoSmithKline Biologicals' combination hepatitis A and hepatitis B vaccine (Twinrix®: 720 EL.U of hepatitis A antigen and 20 mcg of hepatitis B surface antigen per mL) administered on a 0, 1, 6 month schedule by intra-muscular injection compared with concurrently administered monovalent vaccines (Havrix®: 1440 EL.U/mL on a 0, 6 month schedule and Engerix-B®: 20 mcg/mL on a 0, 1, 6 month schedule) in healthy adults 18 years of age and older GlaxoSmithKline Protocol: 49653/183: A 24-Week Randomized, Double-blind, Double-dummy, Multicenter Study to Evaluate the Safety, Efficacy, and Tolerability of Modified Release Avandia® (8mg OD) and Immediate-Release Avandia® (4mg BD) in Patients with Type 2 Diabetes Mellitus GlaxoSmithKline Protocol BRL 49653/330: A Randomized, Double Blind, Placebo Controlled, Parallel Group Study to Assess the Safety and Efficacy of Three Dose Levels of Rosiglitazone Maleate in the Treatment of Chronic Plaque Psoriasis GlaxoWellcome Protocol S3B300020: A 24-Week, Randomized, Open Label Study of Health Care Resource Use, Quality of Life, and Productivity with Alosetron 1mg Twice Daily Versus Traditional Therapy in Females with Non-Constipated Irritable Bowel Syndrome Hennepin County Medical Center A randomized trial comparing cyclosporine with cyclophosphajide plus prednisone in the treatment of progressive membranous glomerulonephritis Hoechst Marion Roussel Protocol HMR 3647A/3009: A Double-Blind, Multicenter, Randomized, ActiveControlled, Two-Arm Parallel-Group Comparative Study of the Efficacy and Safety of Oral HMR 3647(800mg once daily) for 7 to 10 Days versus Oral Trovafloxacin 9200 mg once daily) for 7 to 10 days in the Treatment of Community-Acquired Pneumonia in Adults Hoescht Protocol #403: A multicenter, randomized evaluator blind, comparative study of the efficacy of cefotaxime sodium (Claforan) in the treatment of loser respiratory tract infections with q12h and q8h dosing regimens Hoffman La-Roche Ltd. Protocol MF-4411: Multicenter, Double-Blind, Placebo-Controlled, Randomized Study on the Efficacy and Safety of Ibandronate During Three Years' Treatment in Patients with Post-Menopausal Osteoporosis and Vertebral Fractures using a Continuous Oral (2.5mg daily) and an Intermittent Oral (20mg every 2nd day for 24 days every 3 months) Dosing Regimen Johnson & Johnson Pharmaceutical Protocol RIS-USA-232: Efficacy and Safety of a Flexible Dose of Risperidone Versus Placebo in the Treatment of Psychosis in Alzheimer's Disease Knoll Pharmaceutical Company Protocol DO-109: Safety and Tolerability of Long-Term Administration of Dilaudid SR (Hydromorphone HCl) Knoll Pharmaceutical Company Protocol DO-104: A Repeated-Dose Evaluation of Analgesic Use and Safety of Dilaudid SR (Hydromorphone HCl) in Patients with Chronic Cancer Pain Knoll Pharmaceutical Company Protocol DO-105: A Repeated-Dose Evaluation of Analgesic Use and Safety of Dilaudid SR (Hydromorphone HCl) in Patients with Chronic Non-Malignant Pain Merck & Company Protocol MK-793A: A Randomized, Placebo-controlled, double-blind, parallel study comparing the antihypertensive efficacy and safety of diltiazem extended release (ER)(MODS-A 12 hour formulation) monotherapy, enalapril monotherapy and the combination of diltiazem ER and enalapril in patients with mild to moderate hypertension Merck & Company Protocol MK-218A: A Randomized, Double-blind, placebo-controlled, Multicenter study of the safety and antihypertensive efficacy of concomitant treatment with felodipine ER and enalapril in patients with mild to moderate essential hypertension Merck Protocol 037-01/ERT516: A Prospective, Multicenter, Double-Blind With InHouse Blinding, Randomized, Comparative Study to Evaluate the Efficacy, Safety, and Tolerability, of Ertapenem Sodium Versus Piperacillin-Tazobactam in the Treatment of Complicated Intra-Abdominal Infections in Hospitalized Adults Merck-Schering Plough Protocol P02134: Long-Term, Open-Label, Safety and Tolerability Study of SSCH 58235 in Addition to Simvastatin in Patients with Primary Hypercholesterolemia Who Have Previously Completed the 12-Week DoubleBlind Study (Protocol Nos. P00679 or P00680) National Heart, Lung, and Blood Institute The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Novartis RE: Protocol CSPP100A2201: A multicenter, randomized, double-blind, placebo-controlled, parallel- group study comparing aliskiren 150 mg, 300 mg, and 600 mg to placebo and irbesartan 150 mg in patients with mild-to- moderate essential hypertension Novartis Protocol CSPP100A2203: A randomized, double-blind, multicenter, multifactorial, placebo-controlled, parallel-group study to confirm the efficacy and safety of aliskiren monotherapy, and evaluate efficacy and safety of combinations of aliskiren and valsartan in hypertensive patients Novartis Protocol CCOX189A2360: A 13-week, multicenter, randomized, double-blind, double-dummy, placebo-controlled, parallel trial of 2 different dose regimens of lumiracoxib (100 mg od and 200 mg od initial dose for two weeks followed by 100 mg od) in patients with primary knee osteoarthritis, using celecoxib (200 mg od) as a comparator Novartis Protocol CCOX189A2360E1: A 39-week, open-label extension to CCOX189A2360, a 13-week, multicenter, randomized, double-blind, doubledummy, placebo-controlled, parallel trial of 2 different dose regimens of lumiracoxib (100 mg od and 200 mg od initial dose for two weeks followed by 100 mg od) in patients with primary knee osteoarthritis, using celecoxib (200 mg od) as a comparator NPS Allelix Corporation Protocol ALX1-11-93001: An 18-Month Double-Blind, Placebo-Controlled, Phase III, Trial with a 12-Month Interim Analysis of the Effect of Recombinant Human Parathyroid Hormone (ALX1-11) on Fracture Incidence in Women with Postmenopausal Osteoporosis Protocol LOFBIV-PNOP-001: A Multicenter, Randomized, Open Label Study to Ortho-McNeil Compare the Safety and Efficacy of Levofloxacin with that of Pharmaceutical, Inc. Imipenem/Cilastatin in the Treatment of Nosocomial Pneumonia Pfizer, Inc. Protocol A1281052: A Phase III, Randomized, Placebo-Controlled, DoubleDummy Study Evaluating the Safety and Efficacy of Oral Ziprasidone vs. Haloperidol and Placebo in In-Patients with an Acute Manic Episode Protocol M/1260/0054: Linezolid for the Treatment of Vancomycin-Resistant Pharmacia & Upjohn Enterococcal Infections: A Double Blind Trial Comparing 600mg Linezolid Inc. Every 12 Hours with 200mg Linezolid Every 12 Hours. Protocol M/1260/0033: Linezolid (PNU-100766) in the treatment of streptococcus pneumoniae pneumonia: an open-label study of intravenously Pharmacia & Upjohn administered linezolid with oral continuation compared with intravenously Inc. administered ceftriaxone sodium followed by orally administered cefpodoxime proxetil Rhone-Poulene Rorer Pharmaceuticals, Inc. Protocol JRV 397: Open Study of Synercid (Quinpristin/Dalfopristin, RP 59500) for Emergency Use (Infections Due to Resistant Bacteria, Treatment Failure or in Treatment-Intolerant Patients) EMERGENCY USE Schering-Plough Corporation Protocol P01416: Long Term, Open-Label, Safety and Tolerability Study of SCH 58235 in Addition to Pravastatin in Patients with Primary Hypercholesterolemia Scios Nova Protocol # 702.315: A Randomized, Double-blind, placebo-controlled, Multicenter, phase III clinical trial to evaluate the safety and efficacy of auriculin anaritide in the treatment of oliguric acute tubular necrosis Protocol 4910F/149: A Double-Blind, Randomized, Placebo-Controlled Study SmithKline Beecham to Assess the Efficacy and Safety of Mupirocin Calcium Nasal Spray, 5% for the Prevention of Recurrent Acute Bacterial Sinusitis Protocol SB278817/003: Cervastatin for Hyperlipidemia After Reaching Menopause (CHARM): A Randomized, Double Blind, Placebo-controlled Parallel Group, 6-Week Evaluation of Cerivastatin 0.4mg and Atorvastatin SmithKline Beecham 10mg in Post-Menopausal Women with Hyperlipidemia and a 46-Week Evaluation in Which Cerivastatin 0.4mg is Compared to Fenofibrate 201 mg for Long-Term Lipid-Lowering and Bone Mineral Density Effects Protocol BRL46394A/Granisetron/513: A Double Blind, Multicenter, Parallel Study Comparing the Efficacy and Safety of Kytril Tablets with Placebo, in the SmithKline Beecham Prevention of Nausea and Vomiting During the Days Following Administration of IV Cyclophosphamide-Based or Carboplatin-Based Chemotherapy in Patients with Malignant Disease Takeda Pharmaceuticals North America Protocol 01-02-TL-375-032: A Phase III Safety Study to Evaluate the LongTerm Effects on Endocrine Function in Adult Subjects with Chronic Insomnia Vasomedical, Inc. The International EECP Patient Registry Yamanouchi USA, Inc. Protocol 1025-007: A 4-day, Double-blind, Placebo-Controlled, Multicenter Study of IV YM087 (CI-1025) to Assess Efficacy and Safety in Patients With Euvolemic or Hypervolemic Hyponatremia Yamanouchi USA, Inc. Protocol 1025-011: A 12-Week, Open-Label Extension to Protocol 1025-006 and Protocol 1025-007 to Evaluate the Safety and Efficacy of YM087 (CI-1025 in Patients with Euvolemic or Hypervolemic Hyponatremia


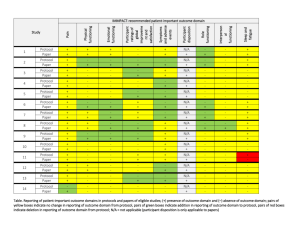



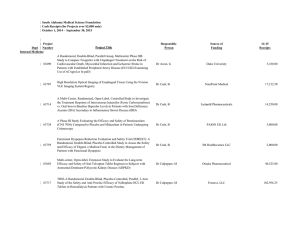

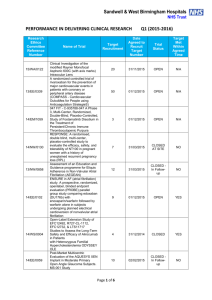
![Quality assurance in diagnostic radiology [Article in German] Hodler](http://s3.studylib.net/store/data/005827956_1-c129ff60612d01b6464fc1bb8f2734f1-300x300.png)
