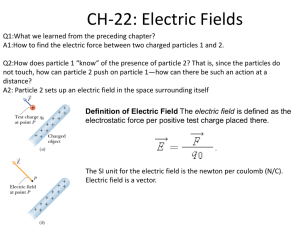
se equation
advertisement
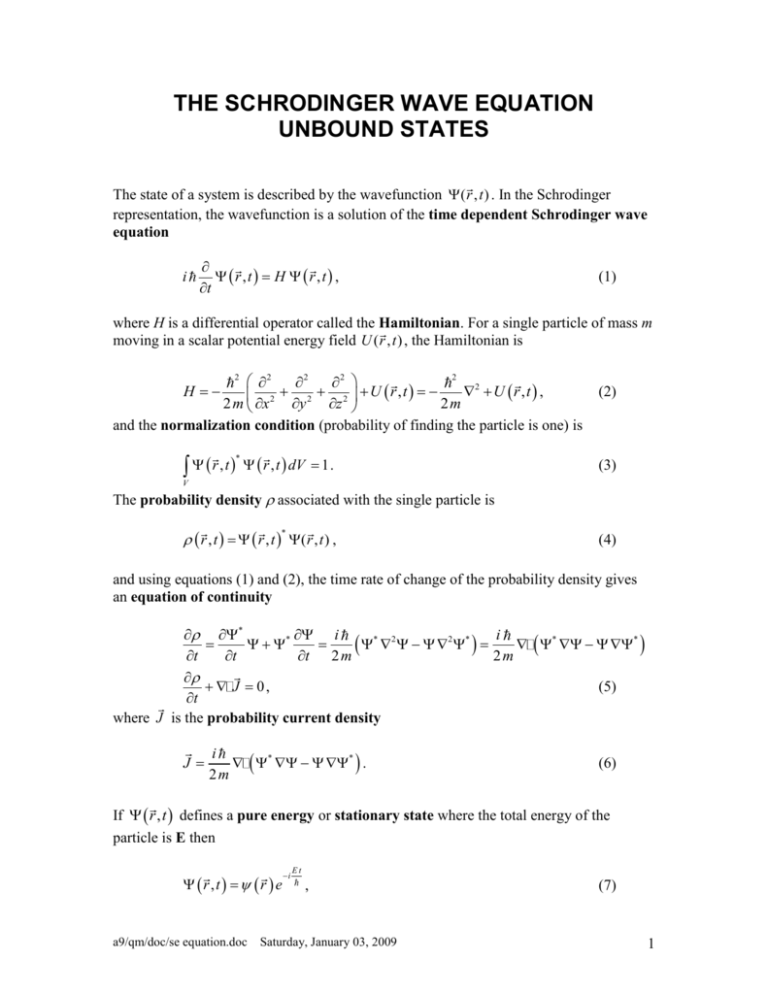
THE SCHRODINGER WAVE EQUATION UNBOUND STATES The state of a system is described by the wavefunction ( r , t ) . In the Schrodinger representation, the wavefunction is a solution of the time dependent Schrodinger wave equation i r ,t H r ,t , t (1) where H is a differential operator called the Hamiltonian. For a single particle of mass m moving in a scalar potential energy field U ( r , t ) , the Hamiltonian is 2 2 2 2 H 2 2 U r ,t 2 U r , t , 2 2 m x y z 2m and the normalization condition (probability of finding the particle is one) is 2 r , t r , t dV 1 . * (2) (3) V The probability density associated with the single particle is r , t r , t ( r , t ) , * (4) and using equations (1) and (2), the time rate of change of the probability density gives an equation of continuity * i i * * 2 2 * * * t t t 2 m 2m J 0, (5) t where J is the probability current density J i * * . 2m (6) If r , t defines a pure energy or stationary state where the total energy of the particle is E then r,t r e a9/qm/doc/se equation.doc i Et , Saturday, January 03, 2009 (7) 1 and the time dependent Schrodinger equation (1) reduces to the time independent Schrodinger equation H r E r . The wavefunction is not represented by the equation r,t r e i (8) Et , because this equation implies that the total energy of the particle is negative. RECTILINEAR MOTION OF A STREAM OF NON-INTERACTING PARTICLES Free Particles For a single particle moving along the x-axis as a free particle with total energy E and zero potential energy, U(x) = 0, the time dependent Schrodinger equation is 2 x, t x, t , t 2 m x 2 and has solutions of the form (9) i x, t ( x ) e i Et Ae i 2 mE x e i Et ( x ) Ae i 2 mE x . (10) defines the total energy of the particle E (energy eigenstate) and t 2 the spatial operator defines the momentum of the particle p 2 m E 2 m x 2 (momentum eigenstate). The total energy of the particle E determines the following parameters describing the particle and its corresponding de Broglie wave The time operator i total energy E kinetic energy K 12 m v 2 E (E = K + U = K since U = 0) 2E m p mv k 2 m E particle velocity v particle momentum wave number (propagation constant) k a9/qm/doc/se equation.doc Saturday, January 03, 2009 2m E 2 de Broglie wavelength frequency f period T h E 2 h h k p 2m E E h angular frequency 2 f phase velocity v phase f k E E 2m d 2E v particle dk m 2 v phase group velocity vgroup vgroup v phase vgroup probability current density (independent of time) k * J ( x ) ( x ) v particle * ( x ) ( x ) . m The solution can be expressed as a monochromatic plane wave by x, t Aei k x t , (11) where A is the amplitude of the wave. The + sign represents a wave traveling in the +x direction and the – sign for traveling in the –x direction. The concept of a monochromatic plane wave is an idealization and does not represent a real physical situation. The wave extends from - to + and there is an infinite stream of particles each with energy E and therefore the system has infinite energy. It is assumed that the total energy of the particle E is exact. The momentum is p 2 m E and hence the momentum is precisely defined. Therefore the particle is not localized and its position is completely uncertain. The wavefunction x, t can not be normalized and * ( x ) ( x ) can not represent the probability density. However, it can be interpreted as the particle density of a stream of non-interacting particles each moving with total energy E and momentum p k . a9/qm/doc/se equation.doc Saturday, January 03, 2009 3 In the S.I. system of units: the wavefunction (x) has units of m-0.5; * ( x ) ( x ) has units of m-1; the velocity vparticle has units of m.s-1; and the current probability density J has units of s-1. Therefore the current probability J can be interpreted as the number of particles n passing a point along the x-axis per second J n k * ( x ) ( x ) v particle * ( x ) ( x ) . m (12) For a uniform particle beam, the amplitude A is related to the intensity of the beam. The number of particles NL on average in a length L is xL xL x x NL * ( x) ( x) dx A2 dx A2 L Therefore, A2 can be interpreted as the number of particles on average per meter. The time independent Schrodinger equation in the case of rectilinear motion in a potential energy field U(x) is 2 x U ( x ) E x 0 . 2 m x 2 2 If the potential energy U(x) has a finite discontinuity at x0 so will (13) 2 x but x and x 2 x need to be continuous to give physically acceptable solutions. x a9/qm/doc/se equation.doc Saturday, January 03, 2009 4 MATLAB – MOTION OF FREE PARTICLE plane_wave_A.m The m-script plane_wave_A.m animates the solution of the time dependent Schrodinger equation for a stream non-interacting particles (electrons) moving along the x-axis with total energy E per particle. Examine and run the m-script for the default values (E = 100 eV). Make sure you understand what the m-script tells you and how the calculations are performed. To understand the motion of the particle it is necessary to calculate and plot both the real and imaginary parts of the wavefunction. It is also useful to display how the phase of the wavefunction evolves with time. The time independent wavefunction x is only calculated once and the time development of the wavefunction is then given by the multiplication of x by e form i Et e i t as described by equation (11) expressed in the x, t Ae i k x ei t . For particles with energy E = 100 eV, the Matlab graphical output and numerical output are: Wavefunction: Stream of non-interacting particles 100 psi ( m -0.5 ) Investigations real imag 0 phase ( rad ) -100 -2 -1 0 x (m) 1 2 x 10 -10 2 1 wavelength: phase cycles through 2 0 -2 x 10 -1 0 x (m) 1 2 x 10 -10 10 -1 J (s ) 10 5 constant probability current density for free particles 0 -2 -1 0 x ( m) -------------------------------------------------------------------------------------------- a9/qm/doc/se equation.doc Saturday, January 03, 2009 1 2 x 10 -10 5 Paramaters for stream of non-interacting particles and associated de Broglie matter wave -------------------------------------------------------------------------------------------Energy of each particle, E = 1.000e+002 eV Kinetic energy of each particle, K = 1.000e+002 eV Momentum of each particle, p = 5.402e-024 N.s Velocity of each particle (group velocity of de Broglie wave), v_gp = 5.931e+006 m/s Wave number of de Broglie wave, k = 5.12e+010 rad/m de Broglie wavelength, lambda = 1.227e-010 m Angular frequency of de Broglie wave, omega = 1.518e+017 rad/s Frequency of de Broglie wave, f = 2.417e+016 Hz Period of matter wave, T = 4.138e-017 s Phase velocity of de Broglie wave, v_ph = 2.964e+006 m/s Amplitude of de Broglie wave, A = 1.000e+002 m^(-0.5) Average number of particles per meter along x-axis, N = 1.000e+004 Number of particles passing a point along x-axis per second, n = 5.931e+010 /s Exercises 1. 2. 3. 4. Run the program a few times and change the animation time. Comment on each graph. Calculate “manually” each of the parameters in numerical output to verify that they are correct. Predict the changes in the parameters when the total energy of each electron is increased from 100 eV to 400 eV. Check your predictions. Use the data cursor and the ginput Matlab command to estimate the wavelength from the (x) graph and the phase angle graph. Do the estimates agree with the theoretical value? a9/qm/doc/se equation.doc Saturday, January 03, 2009 6 Stream of particles incident at a potential energy step with E > U0 Consider the motion of a stream of non-interacting particles in a potential energy field U(x) where in region 1, U(x) = 0 for x < 0 and in region 2, U(x) = U0 (constant) > 0 and U0 < E. energy E I U = U0 R T x=0 U=0 +x Fig. xx. The total energy E and potential energy U(x) function for an electron incident upon a potential step with step height less than the total energy. The time independent Schrodinger equation (12) is 2 x k 2 x 2 x region 1 U(x) = 0 x<0 k 2 k12 region 2 U(x) = U0 x>0 k 2 k2 2 2m E 2 2 m (E U0 ) 2 This corresponds to the classical case where each particle has sufficient energy to surmount the step potential energy barrier and pass into region 2. The step barrier is a repulsive for electrons, hence the kinetic energy of the electrons crossing the step is reduced (K = E – U0). Therefore, the momentum of each electron is smaller and the de Broglie wavelength is longer. We can consider the time dependent wave function to be region 1 x<0 region 2 x>0 1 x, t AI ei k1 x AR e i k1 x e i t 2 x, t AT ei k2 x e i t where 1 x, t represents the superposition of the incident wave (amplitude AI) and the reflected wave (amplitude AR) and 2 x, t defines the transmitted wave. The boundary conditions at x = 0 are 1 0, t 2 0, t 1 0, t 2 0, t x x and these conditions give a9/qm/doc/se equation.doc Saturday, January 03, 2009 7 k1 AI AR k2 AT AI AR AT and hence AR k1 k2 AI k1 k2 AT 2 k1 AI k1 k2 or (14) AI k1 k2 AT 2k1 AR k1 k2 AT 2k1 Normally one specifies the amplitude of the incident wave. However, in the following Matlab simulations, it is more desirable to specify the amplitude of the transmitted wave AT. The number of particles incident nI, reflected nR and transmitted nT per second at the barrier (x = 0) are determined from equation (12) where nI = nR + nT k nT 2 AT 2 m 2 2 k k k nI 1 1 2 AT 2 m 2k1 k k k nR 1 1 2 AT 2 m 2k1 2 2 (15) k k k k nI v1 1 2 AT 2 nR v1 1 2 AT 2 2k1 2k1 where v1 and v2 are the speeds of the particles in regions 1 and 2 respectively The coefficient of reflection R and coefficient of transmission T (R+T = 1) are thus given by nT v2 AT 2 2 k k v v n R R 1 2 1 2 nI k1 k2 v1 v2 2 T nT 4 k1 k2 4 v1 v2 2 2 nI k1 k2 v1 v2 (16) Stream of particles incident at a potential energy step with E < U0 a9/qm/doc/se equation.doc Saturday, January 03, 2009 8 k ( rad/s ) 6 E = 100 eV > U 0 4 k2 2 4 (m) Potential Step Barrier 10 k1 0 0 x 10 20 40 60 80 6 100 -9 1 2 2 0 v ( m/s ) x 10 0 x 10 20 40 60 80 100 6 v1 4 v2 2 0 0 Fig. xx. 20 <--- large step 40 60 E - 0U( eV ) 80 small step --> 100 Variations in k, and v with the height of the step. step_potential.m a9/qm/doc/se equation.doc Saturday, January 03, 2009 9 Potential Step Barrier E = 100 eV > U0 100 A ( m-0.5 ) AI AR 50 0 particles n / s 6 0 x 10 20 40 60 100 10 nI 4 nR 2 nT 0 0 20 40 60 1 R and T 80 80 100 R T R+T 0.5 0 0 Fig. xx. 20 <--- large step 40 60 E - 0U( eV ) 80 small step --> 100 Variations in A, n, R and T with the height of the step potential. step_potential.m a9/qm/doc/se equation.doc Saturday, January 03, 2009 10 a9/qm/doc/se equation.doc Saturday, January 03, 2009 11