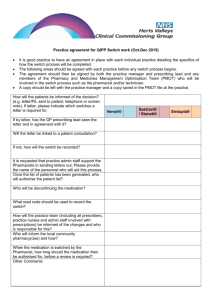
Who should be able to prescribe the drug?
advertisement

New Drug application for: (D&T Application Form 2013) Drugs & Therapeutics Committee (D&T) New Drug Application Joint Primary and Secondary Care – Drug Application This form is to be used for all applications for a new drug, a new formulation, use of samples, or new use of an existing drug. Applications will be discussed by the Drugs and Therapeutics Committee if they are completed electronically in full and come from a Consultant or, in exceptional cases from a non-medical prescriber or from a pharmacist. All sections in this form must be completed and sent electronically and will not be considered until received fully completed electronically. The grey shaded areas give guidance for completion. The Medicines Information Team can assist with the collection and summary of unbiased information, but it is up to you to ask them for help. They can be contacted on extension 6246 or via email to ‘Medicines Information Pharmacists’. All applicants will be invited to the next available meeting of the D&T. Applications might not be considered if the applicant or a suitable representative is not present at the meeting, at the Chairman's discretion. The completed form must be sent electronically to Omar Ali. A copy must also be printed and signed by applicant and the Lead Clinician and posted For The Attention of, Omar Ali, Formulary Development Pharmacist, East Surrey Hospital (Pharmacy Department) Consultant…………………………………………………………………………………………… Speciality……………………………………………………………………………………………. Drug Details Enter drug name (generic or approved name), form, strength What are the indications? Give specific indications with the dose/frequency and duration Please indicate any unlicensed indications Page 1 of 7 New Drug application for: (D&T Application Form 2013) What are the main risks and contra-indications for the drug? What are the CLINICAL RISKS/ADVERSE EVENTS to the patient? Are there any TRUST/CLINICAL GOVERNANCE ISSUES that you would like raise regarding this application? For injection products – Which of the following risks are present for this drug? Y/N Therapeutic risk There is a significant risk of patient harm if the injectable medicine is not used as intended. Use of a concentrate Further dilution (after reconstitution) is required before use, i.e. slow iv bolus not appropriate. Complex calculation Any calculation with more than one step required for preparation and/or administration, e.g. microgram/kg/hour, dose unit conversion such as mg to mmol or % to mg. Complex method More than five non-touch manipulations involved or others including syringe-to-syringe transfer, preparation of a burette, use of a filter. Reconstitution of powder in a vial Where a dry powder has to be reconstituted with a liquid. Use of a part vial or ampoule, or use of more than one vial or ampoule Examples: 5ml required from a 10ml vial or four x 5ml ampoules required for a single dose. Use of a pump or syringe driver All pumps and syringe drivers require some element of calculation and therefore have potential for error and should be included in the risk factors. However it is important to note that this potential risk is considered less significant than the risks associated with not using a pump when indicated. Use of nonstandard giving set/device required Examples: light protected, low adsorption, in-line filter or air inlet. Total number of product risk factors Six or more risk factors = high-risk product (Red). Risk reduction strategies are required to minimise these risks. Three to five risk factors = moderate-risk product (Amber). Risk reduction strategies are recommended. One or two risk factors = lower-risk product (Green). Risk reduction strategies should be considered. Page 2 of 7 New Drug application for: (D&T Application Form 2013) Why is this drug better than other drugs currently used Please insert a referenced summary of the clinical information, NOT drug company literature, in support of your application. (Applications with obviously cut and pasted drug company information may be rejected before reaching the committee) What are the research based benefits of this drug? Are there any disadvantages compared to other drugs currently available? References: Proposed place in therapy 1. Where is the drug's place in therapy (refer to the evidence listed above)? 2. Will it be first / second / third line treatment (what’s the evidence that supports this place?) 3. What patient criteria should be used for using the drug? (Please attach a treatment protocol or guideline) 5. Does your service have the capacity to use this drug in the current service model? Yes / No 6. What have you done to agree a new service model with the commissioners so that you can use this drug? Page 3 of 7 New Drug application for: (D&T Application Form 2013) What is the predicted usage of this drug? 1. Give the prevalence rates for indicated treatment: 2. The proportion of patients expected to receive the drug: 3. The number of patients predicted to be involved per month and per year: 4. How many patients do you have who could be treated now? 5. Is there likely to be an increase in usage over the next 12-36 months – please provide an estimate 6. Which other Consultants are likely to want to use this drug? 7. Have you liaised with the Consultants listed above? Please provide evidence of their support for this application. What information about the drug will you give patients? For unlicensed medicines/uses please provide a Patient Information Sheet. attached not attached What is the cost of the drug? Provide cost per month: How does this compare to the products currently available? Page 4 of 7 New Drug application for: (D&T Application Form 2013) What does this new therapy replace? 1. Is this drug a new class or does it represent one of a family of drugs? 2. Does this therapy replace another drug or a procedure? 3. Does this drug imply creation of a new service/procedure? QIPP How will this intervention improve QUALITY of patient care? In what way is this treatment INNOVATIVE? (how will the NHS do things differently by using this drug?) What does this treatment PREVENT? And how can we measure Patient Outcomes? What is the PRODUCTIVITY gain to the NHS? (How does this treatment pay for itself?) Page 5 of 7 New Drug application for: (D&T Application Form 2013) What will the effect be on Primary Care prescribing? Do you expect to transfer prescribing and monitoring of this drug to GPs? (If no, go to the next section) How long will you prescribe this drug before you expect GPs to take over prescribing? What monitoring will be required by the GP? (include frequency and criteria for action) What special precautions will the GP need to be aware of when taking on prescribing? If there is any specialist monitoring required by GPs you must attach a shared care guideline when you make this application (example shared care link): Shared care guideline attached What personal experience do you have of using the drug? e.g. clinical trials, experience from a previous hospital Who should be able to prescribe the drug? Consultant prescription only; Specific clinical team(s) - please specify; All grades, all teams. Non-Medical / Dependant Prescribers / Patient Group Directions / Supplementary Prescribing Page 6 of 7 New Drug application for: (D&T Application Form 2013) Declaration Do you have any staff or equipment paid for by the company, or any other financial interest in the company which makes the drug? (including share holdings, lecture commitments, or any part-time work on behalf of the company) I can / cannot attend the next D&TG meeting (see the pharmacy intranet site for details) I am familiar with the data on the drug for which I am applying. I am prepared to attend the next available D&TG meeting to support this application. I will not prescribe the drug (or in the indication described) until I have gained approval from the D&TG and appropriate funding approval. I understand drugs with a significant impact on primary care will need to be agreed by commissioning groups before GPs will take over prescribing. ALL FIELDS MUST BE COMPLETED OTHERWISE APPLICATION FORMS WILL BE RETURNED Signed …………………………………………………………. Date ……………………………………….. Please state e-mail address (your own or your secretary's) for correspondence: …………………………….. The Lead Clinician By signing this section the Lead Clinician is accepting the responsibility and accountability for the impact of the application on the quality, safety, patient experience and cost to their service. Please tick boxes as appropriate I do support the prescribing of this drug as described in the above application with the stated restrictions I have identified funding for the new drug. Signed …………………………………………………………. Date ……………………………………….. Lead Clinician for: ………………………………………………………………………………………………………. Send this completed form with: Supporting information and a product data sheet or Summary of Product Characteristics To: Omar Ali , Formulary Development Pharmacist, Pharmacy Department, East Surrey Hospital. Page 7 of 7