Vit K for supratherapeutic INR
advertisement

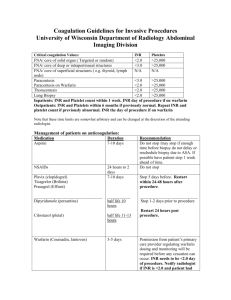
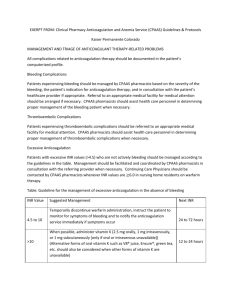
Oral Vitamin K Versus Placebo to Correct Excessive Anticoagulation in Patients Receiving Warfarin Scenario: 73m w/ hx of AF (goal INR 2-3 on 5mg Warfarin, rate controlled on bb therapy), DM, HTN presents to clinic for routine f/u and on review of his labs you notice an INR of 7.8. He has no c/o, vitals at goal, and no hx of recent bleeding. What do you decide to do? Do you give Vitamin K, and if so what dose…or do you let him ride down, withholding his Warfarin dose until his INR is in the therapeutic range? STUDY/METHODS: Purpose: To evaluate whether low-dose vitamin K reduces bleeding events over 90 days in pts with Warfarin-associated coagulopathy w/o increasing risk for thromboembolism. Design: Multicenter, randomized, placebo controlled trail encompassing 14 anticoagulation clinics in Canada, US, and Italy. Inclusion Criteria: Patients receiving therapy anticoagulation in outpatient anticoagulation clinic with their most recent INR b/t 4.5 -10.0 w/n last 24hrs, their target Goal INR 2.0-3.5, and not currently bleeding. Exclusion Criteria: Patients < 18yrs, life expectancy <10 days, indication for acute normalization of INR (surgery), known liver disease, hx of major bleeding event w/n 1 month, known bleeding d/o, received thrombolytics w/n 48hrs, had a plt count < 50, allergic to vitamin K, unable to take oral meds, or could not return to f/u. Intervention: 724 pts w/ INR b/t 4.5 – 10.0 were selected and randomized to two groups, one to receive 1.25mg of Warfarin and one to receive placebo (placebo with filler and indistinguishable from actual pill). Patients, clinicians, and adjudication committee were all blinded to treatment. Patient’s had f/u to measure INR at 1,3,7,14,28, and 90 days. Once the INR had reached goal range, further INR management was left to the clinician’s discretion. Of note: in 2 centers, the INR was monitored outside the clinical center and study drug had to be shipped by courier to the patient’s house and confirmation was of taking the drug was received over the phone (same day). Measurements: Primary Outcome – All bleeding events. Secondary Outcomes – thromboembolism and death **Study was powered to detect an absolute 5% risk reduction in the frequency with all bleeding events with vitamin K. A sample size of 330 patients per group was required to have power of 80% to detect differences w/ a 2-sided p-value of 0.05. IS THE STUDY VALID? (FRISBE PNEUMONIC) Follow-up – YES! (see figure pg.3) Only 12 lost to f/u (assumed to not have bleeding events) A good study will have >80% follow-up rate. Randomization – YES! Intention to treat (analysis of the patients w/n the treatment group they were assigned, regardless of whether they received the actual therapy or not) – YES! Similar groups – YES! (see table 1) Blinding – MAYBE… (clinicians could deduce patient group based on quickness of INR reduction) Equal treatment aside from experimental intervention – YES! RESULTS: 1. Primary Outcomes – All Bleeding Events (see Table 2) Vitamin K group 56 (bleeding event) /347 Placebo 60 (bleeding event) /365 Control Event Rate (CER) 60/347 = 16.4 Experimental Event Rate (EER) 56/347 = 16.1% Relative Risk (RR) EER/CER = 98% Relative Risk Reduction (RRR) 1 – RR = 2% Absolute Risk Reduction (ARR) CER – EER = 0.3% Number Needed to Treat (NNT) 1/ARR = 333 2. Secondary Outcomes (see Table 3) – Post-Hoc Analysis - Underpowered for Major Bleeding d/o (not enough occurrences) 2ndary outcomes - Death and thromboembolism were not significant different b/t the two groups. 3. Subgroup Analysis: Patients greater > 70yrs had 10/13 Major Bleeding Events noted in the study. DISCUSSION: Based on this study it appears that low dose Vitamin K reduces the INR in patients over-anticoagulated on Warfarin therapy, but had little effect on clinical outcomes and did not significantly reduce bleeding events. In “well to do” outpatients with INR’s b/t 4.5-10.0 withholding Warfarin therapy may be all that is needed. ** After-Thoughts * no mention on patient concomitant use of anti-platelet agents (ASA/Plavix) during this trial * duration of warfarin therapy prior to trial (bleeding rate 2x higher in 1 st month of therapy) * 90 day f/u after administration of 1.25mg Warfarin…dietary constituents can best that. * clinicians could potentially deduce vitamin K groups based on rapid INR correction and might manage these patients differently vs. and elderly >70 patient w/ high INR. * Take subgroup analysis w/ a grain of salt. Most studies enroll just enough patients to ensure the primary hypothesis can be tested. As you reduce the N# for subgroup analysis, power is greatly reduced. Is this study applicable to our patients? Hospital vs. Outpatients Back to our patient: What do you decide to do? Chest Guidelines 2008 on Overanticoagulation INR 5.0 – 9.0 allows clinicians the option to either withhold Warfarin therapy for 1-2 doses or to administer a low dose vitamin K 1.0 – 2.5mg. Does this paper change our school of thought?