Hege Brun-Hansen (Norway) Diagnosis of Red blood cell parasites
advertisement

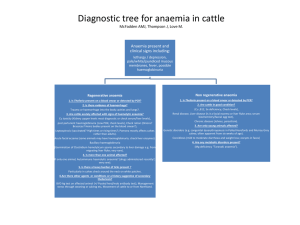
Diagnosis of red blood cell parasites. Brun-Hansen, Hege Norwegian School of Veterinary Science, Central Laboratory, P.O.Box 8146, 0033 Oslo, Norway hege.brun-hansen@veths.no Babesia spp. Several species of Babesia cause haemolytic anaemia and thrombocytopenia in domestic animals. Babesia canis and Babesia gibsoni are pathogenic in dogs and wild canids. Three distinct subspecies of B.canis exist: B.canis vogeli, B.canis canis, and B.canis rossi. B. canis rossi, which occurs in South Africa, tends to cause the most severe disease. B canis canis occurs in Europe and Asia, whereas B.canis vogeli occurs in the United States and tropical and subtropical areas B.gibsoni is a small babesia that occurs in Asia, Africa, Middle East, the US and Australia. Babesia gibsoni has also been isolated from a dog in Spain. B.gibsoni was previously thought to be the only small babesia parasitizing dogs, however genetic sequencing has revealed that there are at least three distinct subtypes of small babesia affecting dogs. Babesia bovis, Babesia bigemina and Babesia divergens cause haemolytic anaemia in cattle. B.bovis and B.bigemina occur worldwide in tropical and subtropical regions. B divergens occurs in cattle in Europe including Scandinavia and North Africa. The aetiological agents of equine babesiosis are Babesia equi and Babesia caballi. B. equi has been found to be more closely related to Theileria spp and has been renamed Theileria equi. Four species of Babesia can infect domestic and wild felids; B.cati, B.felis, B.herpailuri and B.panthera. Feline babesiosis is reported in South America, Africa and India. Babesiosis in domestic small ruminants is due to, at least, three species; B.ovis, B.motasi and B.crassa. Among these B.ovis is the most pathogenic Diagnosis of babesiosis includes microscopic examination of blood smears, serological testing and molecular analyses. Microscopic examination of Romanowsky-type stained blood smears can confirm infection, but not accurately determine identity of the babesial organism. Diagnosis may be complicated because clinically affected animals do not always have organisms visible on blood smears. Many animals that survive the infection become chronic carriers with very low numbers of infected erythrocytes that are extremely difficult to impossible to detect microscopically. Serological tests include complement fixation test (CFT), immunofluorescent antibody test (IFAT) ,enzyme linked immuno-sorbent assay (ELISA) and rapid conglutination test (RCT). The serological tests are not sensitive enough to detect carrier animals. PCR tests are sensitive tests that also are able to differentiate between different species. Seminested PCR testing can detect parasitaemia levels that are more than 1000 times lower than the approximate 0.001% parasitaemia detectable by light microscopy. PCR tests have been developed for several species.Reverse line blot hybridisation test gives the possibility of detecting all protozoan parasites that could possibly be present in the blood of a carrier animal. Theileria spp Two Theileria species are pathogenic for cattle. Theileria parva causes East Coast fever in Africa, and Theileria annulata the cause of tropical theileriosis in the Mediterranean basin, Middle East and Asia. Haemolytic anaemia with hemoglobinuria and icterus occurs in cattle infected with Theileria annulata. There are at least two recognised species that are pathogenic in sheep and goats. These are Theileria lestoquardi and the fairly recently described Theileria species China 1. T. lestoquardi is endemic in Southern Europe, North Africa and Asia whereas T.sp.China 1 has only been identified in China. There are also several Theileria spp that are regarded as non pathogenic. Theileria has a tissue phase as well as blood phase. First sporozoites infect mononuclear cells where schizonts are developed these schizonts release merozoites, which infect red blood cells. The schizogony was thought to occur in lymphocytes but more recent reports show that schizonts of T.annulata are found in monocytes/macrophages.Diagnosis of clinical T.annulata infection in cattle is usually based on the detection of schizonts in lymph node biopsy smears or by finding the piroplasms in erythrocytes. After recovery a long lasting carrier state occurs, in which low numbers of erythrocytes remain infected with the piroplasms. These can be very difficult to detect in stained blood smears. Serological tests include IFAT and ELISA. IFAT is the most widely used diagnostic test but cross reactivity between different Theileria species limits the specificity. An ELISA test adapted for detection of T.annulata has been shown to detect antibodies for a longer period if time than the IFAT. Molecular analyses include PCR and reverse line blot hybridisation. Cyauxzoon felis: Cytauxzoon felis is a protozoan classified within the same family as Theileria. Like theileria, merozoites infect erythrocytes, while a tissue phase, the schizonts, infect and fill macrophages within and surrounding blood vessels throughout the body. The disease is usually fatal. It occurs in the midwestern and southern United States Diagnosis is made by finding the signet-ring shaped piroplasms in blood films relatively late in the course of the disease or by finding the schizonts in macrophages by cytologic examination of spleen, liver, lymph node or bone marrow aspirates. Anaplasma spp.:Three species of Anaplasma are well recognised: A. marginale and A.centrale, which infect cattle, and A.ovis that infects goats and sheep. A. marginale is the most prevalent tick-borne infection of cattle worldwide and causes severe losses for livestock production. Acute anaplasmosis is characterised by a progressive anaemia, weight loss, decreased milk production, abortion, and, in 36% of clinical cases, death. Surviving animals remain persistently infected and serve as reservoirs for ticks to become infested. A.centrale causes only a mild form of diseaseAcute infections can be reliably identified using microscopic examination of blood or organ smears stained with Romanowskytype stain, but carrier cattle is difficult to impossible to detect by this method. CFT has been used extensively for many years but the sensitivity is not good enough to detect carrier cattle. A competitive ELISA using a recombinant antigen termed rMSP5 and MSP5-specific monoclonal antibody has proven very sensitive and specific for detection of Anaplasma-infected animals. A commercial kit is available. PCR-tests have also been developed for detection of Anaplasma infection. Hemotrophic mycoplasmas Hemotrophic mycoplasmas consist of the former Haemobartonella and Eperythrozoon species. These are pleomorphic extracellular Gram-negative bacterial organisms that parasitize red blood cells in a wide range of vertebrate animals. Two organisms have been identified in the cats; the large form, which is named Mycoplasma haemofelis, and a small form named Candidatus Mycoplasma haemominutum. Infection with Mycoplasma haemofelis can cause a potentially fatal clinical syndrome of domestic cats. Clinical signs include anaemia, splenomegaly, fever, lethargy and sometimes icterus. Concurrent disease, immunosuppression, or splenectomy may predispose animals to acute infection. The anaemia is regenerative unless underlying disease inhibits erythropoiesis. Candidatus Mycoplasma haemominutum is less pathogenic and clinical signs may be minor or absent. The most commonly used method to diagnose Mycoplasma haemofelis infection is demonstration of organisms on the surface of erythrocytes by microscopic examination of a good quality blood smear. The organism is 0.5-0.6 μm and may be found singly, in pairs or in chains. The parasitaemia is cyclic and the clearance of parasites from the blood can be rapid resulting in negative cytological findings within less than an hour. No serological test is currently commercially available PCR tests for Mycoplasma haemofelis and Candidatus Mycoplasma haemominutum are commercially available in Europe and United States. Samples should be submitted before antimicrobial therapy, which will cause PCR results to be negative. Other hemotrophic mycoplasmas include M.wenyonii, M.haemolamae and M.haemocanis that are regarded as opportunistic organisms; M.haemosuis, which is pathogenic in very young pigs, and M.haemoovis that may cause anaemia in sheep and goats. References: Boozer A.L, Macintire D.K., 2003.Canine babesiosis. Vet Clin Small Anim Pract, 33: 885-904 Braddock J.A., Tasker S., Malik R., 2004 . The use of real-time PCR in the diagnosis and monitoring of Mycoplasma haemofelis copy number in a naturally infected cat. Journal of Feline Medicine and Surgery, 6: 161-165. Criado-Fornelio A, Gonzalez-del-Rio M.A, Buling-Sarana A, Barba-Carretero J.C, 2003. Molecular characterization of a Babesia gibsoni isolate from a Spanish dog. Veterinary Parasitology,117:123-129. Forsyth, L.M.G, Minns F.C., Kirvar, E, Adamson R.E, Hall F.R, McOrist s, Brown C.G.D, Preston P.M, 1999. Tissue Damage in Cattle Infected with Theileria annulata Accompanied by Metastasis of Cytokine-producing, Schizont-infected Mononuclear Phagocytes. Journal of Comparative Pathology,120: 39-57. Nagore D, Garcia-Sanmartin J, Garcia-Perez A, Juste R.A, Hurtado,A. 2004. Identification, genetic diversity and prevalence of Theileria and Babesia species in a sheep population from northern Spain. International Journal for Parasitology, In press. Nicolaiewsky T.B, Richter M.F, Lunge V.R, Cunha C.W, Delagostin O, Ikuta N, Fonseca A.S, da Silva S.S Ozaki L.S, 2001. Detection of Babesia equi (Laveran 1901) by nested polymerase chain reaction. Veterinary Parasitology 101: 9-21. Schnittger L, Yin H, Qi B, Gubbels, M.Jniemann S, Jongejan F, Ahmed J.S,2004. Simultaneous detection and differentiation of Theileria and Babesia parasites infecting small ruminants by reverse line blotting. Parasitology Research,92: 189-196. Tasker S, Helps C.R, Day M.J, Gruffydd-Jones T.J, Harbour D.A, 2003. Use of Real-Time PCR to Detect and Quantify Mycoplasma haemofelis and “Candidatus Mycoplasma haemominutum” DNA. Journal of Clinical Microbiology,41:439-441. Torioni de Echaide S, Knowles D.P, McGuire T.C, Palmer, G.H, Suarez, C.E, McElwain, T, 1998. Detection of Cattle Naturally Infected with Anaplasma marginale in a Region of Endemicity by Nested PCR and a Competitive Enzyme-Linked Immunosorbent assay Using Recombinant Major Surface Protein 5. Journal of Clinical Microbiology,36: 777-782.