Circulating tumor cells
advertisement

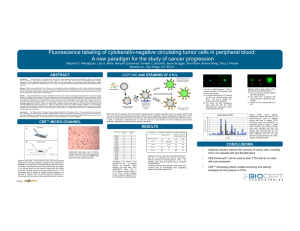
Circulating tumor cells (CTCs) in blood of breast cancer patients: Cytological detection and technical characterization Enrica Bresaola, Mara Jo Miller, Marco Picozzi, Maria Teresa Sandri, Chiara Casadio ______________________ Cytology Unit, Department of Pathology & Laboratory Medicine European Institute of Oncology, Milan, Italy Introduction Circulating tumor cells (CTCs) were described for the first time more than one century ago and their occurrence in the bloodstream fit with the hematogenous theory of metastatization Currently, their detection may play a pivotal role in the prognosis and prediction of therapy efficacy, providing us with insights into the clinical outcome, cell dissemination, drug resistance and treatmentinduced cell death Purpose To assess the feasibility of detecting CTCs in blood samples of breast cancer patients using the Thin Prep® cytological preparation after a concurrent analysis with the CellSearch System (Veridex LLC, Warren, NJ) To further characterize these cells according to estrogen receptor immunoreactivity and Her-2/neu gene status evaluation by fluorescence in situ hybridization (FISH) analysis Materials and Methods 7.5 ml of whole blood were drawn from breast cancer patients into the CellSave Preservative Tube containing a cellular preservative and processed within 72 hours The CellSearch Profile kit was then utilized to separate the CTCs by treatment with iron particles coated with antibodies against the Epithelial Cell Adhesion Molecule (EpCAM) for capturing CTCs CTCs were then magnetically separated out and concentrated into a remaining aliquot of 1ml Materials and Methods This aliquot was centrifuged at 1700 rpm for 7 minutes and the supernatant discarded The pellet was then added directly into the Preservcyt® vial for subsequent processing of ThinPrep slides The slides were colored with H&E and evaluated microscopically Estrogen receptor immunostaining and FISH analysis were carried out according to previously refined laboratory methods Results A total of 106 blood samples, where the CellSearch System obtained CTCs, were further evaluated cytologically Of these, 60 were negative and 46 were positive for malignant cells (range: 1-615) (Figure 1) Figure 1 Results Immunocytochemistry for estrogen receptor was performed in 7 samples and only one case showed ERpositive tumor cells +ve cell -ve cell 10 cases were analyzed by FISH: 7 cases had no amplification while in 3 cases no more cells were detected Conclusions Cytological detection of CTCs in blood specimens from breast cancer patients can be useful in providing samples for testing predictive indicators of prognosis and clinical response during therapy