Virus vectoring ability of a Xiphinema americanum
advertisement

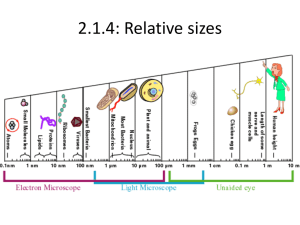
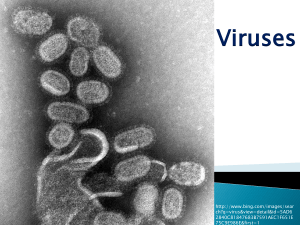
By: Charlie Ta Mentor: Dr. Inga Zasada United States Department of Agriculture: Agricultural Research Services Plant-parasitic nematodes cause $100 billion in crop loss annually worldwide; $10 billion in the U.S. (blueberries, red raspberries, and wine grape industry) Plants affected by X. americanum or nepoviruses become(s) necrotic, yield is reduced, and plant mortality can occur Currently few methods exist to control nematodes or remediate the diseases they transmit Regulations by the U.S. Environmental Protection Agency will soon limit/ban pre-plant fumigation which has traditionally been used to eradicate virustransmitting nematodes head tail Microscopic roundworm(s) that parasitize plants Migratory ectoparasite Acquire and transmit nepoviruses such as Tomato Ringspot Virus (ToRSV) and Tobacco Ringspot Virus (TRSV) with their odontostyle Nematode-transmitted virus with polyhedral particles Type IV virus under the Baltimore classification system (positive sense odontostyle single-stranded RNA that directly translates into protein) Acquisition of virus occurs during feeding and binds to the surface of the odontostyle Viruses are lost when nematodes molt Reverse Transcriptase Quantitative Polymerase Chain Reaction (RT-qPCR) A RT-qPCR can be used for the detection of ToRSV in X. americanum at low concentration levels. Virus detection using RTqPCR allows for a detailed study of nematode-virus interactions. Enzyme-Linked Immunosorbent Assay (ELISA) Steps: 1 2 3 4 5 http://homepage.usask.ca/~vim458/virology/studpages2007/Maura_Tim/For%20Maura%20%20Virology%20website%20assignment/elisa.jpg The coloration occurs due to adding p-nitrophenyl phosphate. Could a RT-qPCR method be developed to enable detection of small concentrations of ToRSV? A RT-qPCR method will be proficient in detecting low concentrations of viruses. X. americanum acquires ToRSV within a week of feeding on a virus infected host. This time period allows for additional virus particles to be acquired by the nematode I. II. Develop and Probe: optimize the Forward: Reverse: efficiency of a RT-qPCR to detect ToRSV Quantify acquisition and saturation level of ToRSV in X. americanum Methodology Objective I: Development Develop an internal positive control (IPC) for RTqPCR by examining homogeneity of the internal transcribed spacer (ITS) region 1 of X. americanum Design IPC to similar length as the ToRSV primer/probe set for multiplex purposes Analyze the two sets for cross reaction and non target RNA with each other. Examine the thermodynamic compatibility using hybridization software and cross referencing sequence data available on Genbank Validate RT-qPCR method with known virus infected samples. Ensures that our samples have nematodes IPC unsuccessful Individual genetic diversity in the group X. americanum Chromatograph illustrating the heterogeneity within the ITS1 region of rDNA for a single individual X. americanum A single signal becomes multiple signals; We observed this with individuals other than Xiphinema as well Literature suggest phylogenetic studies on nematodes is a common problem Objective I: Efficiency A 1 to 10 dilution series of ToRSV from leaves. 10-1 to 10-10 all amplified 10-1 10-2 10-8 10-9 10-10 10-3 -4 10 10-5 10-6 10-7 Detection of ToRSV in roots 11, 9, 6, and 5 weeks Threshold values of ToRSV from amplification plot Ct Mean of ToRSV in Roots 30 25 20 RNA 15 10 5 0 Inoculation Date Dilution series of ToRSV in Roots Detection of ToRSV in roots was lower than ToRSV in leaves 10-1 10-2 10-3 10-4 10-1 to 10-4 amplified http://mexicanmortarandpestle.net/images/large%20mort ar%20and%20pestle.jpg http://www.tari.gov.tw/tarie/photos/introduction/introductio n_PPD_17.jpg http://www.medicine.virginia.edu/research/c ores/biomolec/images/rt-pcr.jpg http://2010.igem.org/wiki/images/thumb/c/cb/IC_Assay_3 _sept.jpg/300px-IC_Assay_3_sept.jpg http://image.made-in-china.com/2f0j00dCLaFpKzrDos/96-Well-Cell-Culture-Plate.jpg X. americanum Have low fecundity Delicate and sensitive to disturbances Inoculation and recovery of nematodes were low RNA extraction was poor IPC did not work Develop and optimize a RT-qPCR method to detect TRSV in X. americanum Determine the persistency and duration of ToRSV/TRSV within X. americanum by using the developed primers/probes for RT-qPCR Link the genetic variability of X. americanum populations to virus vectoring capabilities as a means to facilitate the development of diagnostic tools Dr. Inga Zasada Amy Peetz Dr. Bob Martin Karen Keller Nola Mosier Ruth Price Dr. Kevin Ahern Howard Hughes Medical Institute Cripps Scholarship