Why Fluorine? - Groupe Charette
advertisement
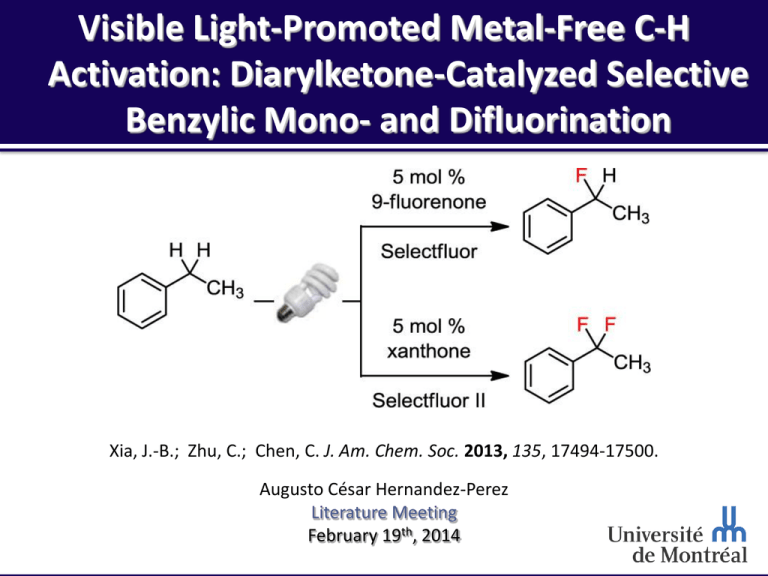
MALLORY REACTION. Visible Light-Promoted Metal-Free C-H Activation: Diarylketone-Catalyzed Selective Benzylic Mono- and Difluorination Xia, J.-B.; Zhu, C.; Chen, C. J. Am. Chem. Soc. 2013, 135, 17494-17500. Augusto César Hernandez-Perez Literature Meeting February 19th, 2014 Outline CARBON RICH MATERIALS AND THE MALLORY REACTION. Pr. Chuo Chen Why fluorine? Mono-fluorination Reaction proposal Difluorination Mechanistic studies Conclusion 2 Pr. Cho Chen CARBON RICH MATERIALS AND THE MALLORY REACTION. Birth: Taipei, Taiwan B.S. degree: National Taiwan University (1995) Ph.D.: Harvard University under the direction of Prof. Matthew D. Shair (2001) Post-doc: Harvard University under the guidance of Prof. Stuart L. Schreiber (2001-2004) Joined the Biochemistry Department at the University of Texas Southwestern Medical Center (2004) Promoted Associated Professor (2010) Award: Southwestern Medical foundation Scholar in Biomedical Research (2004) http://pubs.rsc.org/en/content/articlehtml/2011/cc/c0cc90144j http://www4.utsouthwestern.edu/chuochen/group.htm 3 Research Program 1. Chemical Biology: Synthesis of small-molecule inhibitors of the Hedgehog (Hh) and Wnt signal transduction pathways Mechanistic and medicinal chemical studies of a series of novel Hh and Wnt antagonists 2. Natural product synthesis: Ageliferin Nakiterpiosin Nakiterpiosinone 3. Synthetic methodology development: Palladium-Catalyzed Direct Fonctionalization of Imidazolinone Regiocontrol in MnIII-Mediated Oxidative Heterobicyclizations A Highly Selective Vanadium Catalyst for Benzylic C–H Oxidation A Simple Method for the Electrophilic Cyanation of Secondary Amines http://www4.utsouthwestern.edu/chuochen/group.htm 4 Why Fluorine? – Properties •Special properties: High electronegativity Relatively small size Bond energy/ Bond length / Volume / Å3 van der Waals Pauling radius / Å Electronegativity kcal mol-1 C : 1.70 2.55 C-C : 83 H : 1.20 2.20 C-H : 98 C-H : 1.09 CH3 : 21.26 F : 1.47 3.98 C-F : 116 C-F : 1.41 CF3 : 39.8 O : 1.52 3.44 C-O : 91 C-O : 1.43 CH3CH2 : 38.9 Å (CH3)2CH : 56.2 •C-F bond: Strongest bond with carbon Lenght similar C-O bond Trifluoromethyl (CF3) volume similiar to ethyl (CH3CH2) Bondi, A. J. Phys. Chem. 1964, 68, 441-451. Jeschke, P. ChemBioChem 2004, 5, 570-589. Smart, B. E. J. Fluorine Chem. 2001, 109, 3-11. Banks, R. E. J. Fluorine Chem. 1998, 87, 1-17. Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881-1886. 5 Why Fluorine? – Utility in Different Fields •Useful in medical chemistry: Increase metabolic stability: oxidation by liver enzymes (P450 cytochromes) : block reactive site by the introduction of a fluorine atom Reduces basicity when close to a basic group (better membrane permeability) •Fluorine in nuclear medicine: PET : Positron emission tomography Nuclear medical imaging in vivo 18F tracer has longer half life Radionuclide 11C 13N 15O 18F Half live t1/2 / min 20 10 2 110 •Crop protection: 28% of the halogenated products between 1940-2003 contained fluorine Environmental friendly compare to others halogens •Material industry Fluorinated polymers exhibit interesting properties (high thermal stability, chemical inertness) Böhm, H.-J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. ChemBioChem 2004, 5, 637−643. Phelps, M. E. Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 9226-9233. Jeschke, P. ChemBioChem 2004, 5, 570-589. 6 Okazoe, T. Proc. Jpn. Acad., Ser. B 2009, 85, 276-289. Fluorine Incorporation •Nucleophilic fluorination: Small size of the atom and low polarisability encourages F- to act more like a base rather than a nucleophile Various nucleophilic reagents (F-, S-F reagents) •Electrophilic fluorination: Not easily achieved because fluorine is the most electronegative element Use of N-F reagents (even 5% F2 in N2) •Radical fluorination: Use of N-F reagents Kirk, K. L. Org. Process Res. Dev. 2008, 12, 305-321. 7 Mono-Fluorination – Literature Precedent •Functional group transformation: Nucleophilic fluorination Yadav, A. K.; Srivastava, V. P.; Yadav, L. D. S. Chem. Commun. 2013, 49, 2154-2156. York, C.; Prakash, G. K. S.; Olah, G. A. Tetrahedron 1996, 52, 9-14. Electrophilic / Radical fluorination Cazorla, C.; Métay, E.; Andrioletti, B.; Lemaire, M. Tetrahedron Lett. 2009, 50, 3936-3938. Rueda-Becerril, M.; Sazepin, Chatalova Sezapin, C.; Leung, J. C. T.l Okbinoglu, T.; Kennepohl, P.; Paquin, J.-F.; Sammis, G.M..dav, L. D. S. J. Am. Chem. Soc. 2012, 134, 4026-4029. •Drawbacks: Narrow scope / few substrates Methodology not for large synthesis scale 8 Mono-Fluorination – Literature Precedent •Direct C-H fluorination: Electrophilic / Radical fluorination Bloom, S.; Ross Pitts, C.; Curtin Miller, D.; Haselton, N.; Gargiulo Holl, M.; Urheim, E.; Lectka, T. Angew. Chem., Int. Ed. 2012, 51, 10580-10583. Bloom, S.; Ross Pitts, C.; Woltornist, R.; Griswold, A.; Gargiulo Holl, M.; Urheim, E.; Lectka, T. Org. Lett. 2013, 15, 1722-1724. •Features: Catalytic system Mild reaction conditions Decent scope 9 Reaction Proposal •Key steps: Formation of photoexcited arylketone Benzylic hydrogen abstraction Fluorine atom transfer Regeneration of catalyst Use of visible light No transition-metal used •Photoredox chemistry: Use of visible light Use of transition-metal (Ru, Ir) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322-5363. Tucker, J. W.; Stephenson, C. R. J. J. Org. Chem. 2012, 77, 1617-1622. 10 Photoexcited Arylketone – Literature Precedent •Yang’s report: Acetone in cyclohexane gives cyclohexylpropan-2-ol under UV light •Intramolecular reaction: Norrish-Yang cyclization Intramolecular H abstraction at position and cyclization •Benzophenone: Acts like acetone Known to abstract hydrogen from the triplet state (photo-excited state) Abstracts hydrogen from cylohexane or ethylbenzene (benzylic position) •Drawbacks: Use of UV light (mercury lamp) High dilution conditions Yang, N. C.; Yang, D.-D. H. J. Am. Chem. Soc. 1958, 80, 2913-2914. http://goldbook.iupac.org/N04218.html. Walling, C.; Gibian, M. J. J. Am. Chem. Soc. 1965. 11 Reaction Conditions – Optimization 4,2$/g 76,1$/g 5,7$/g 21,3$/g 49,2$/g •Features: Use of visible light effective with compact fluorescent lamp (cheap!) 9-fluorenone has suitable chromophore for visible light Ir(ppy)3 does not promote benzylic fluorination Not water sensitive but oxygen sensitive Cheap electrophilic fluorine source 12 Benzylic Monofluorination – Scope •Features: Fast reaction with EDG (if too electron rich, side reaction with Selectfluor) Aromatic halides tolerated (no UV light used) 1 and 2 alcohols not compatible MIDA boronate tolerated under reaction conditions 13 Difluorination – Literature Precedent •Baran’s zinc sulfinate salt: Broad scope of nitrogen-rich heterocycle / not possible on benzene ring Good group tolerance Salt commercially available Zhou, Q.; Ruffoni, A.; Gianatassio, R.; Fujiwara, Y.; Sella, E.; Shabat, D.; Baran, P. S. Angew. Chem., Int. Ed. 2013, 52, 3949-3952. Patrick, T. B.; Flory, P. A. J. Fluorine. Chem. 1984, 25, 157-164. York, C.; Prakash, G. K. S.; Olah, G. A. Tetrahedron 1996, 52, 9-14. Fier, P. S.; Hartwig, J. F. J. Am. Chem. Soc. 2012, 134, 5524-5527. •Other methods: Electrochemical difluorination (mixture of products) Few difluorination methods available 14 Benzylic Difluorination – Optimization •Features: Xanthone (C) is electron-rich enough to promote difluorination Selectfluor II effective with xanthone (C) 15 Benzylic Difluorination – Scope •Features: No or less than 5% of monofluorinated product in all cases Aromatic halides tolerated (no UV light used) MIDA boronate tolerated under reaction conditions 16 Mechanistic Studies •Features: Visible light and catalyst are required No reaction in the dark No thermal radical process 9-fluorenone doesn’t act as an energy transfer photosensitizer Reaction works in the presence of a 400 nm long-pass filter •Kinetic isotope effect (KIE) : C-H abstraction in the rate-limiting step 17 Mechanistic Studies •Site preference for reaction : 2 1 3 Electron rich substrates react faster than electron-poor substrates •Gram scale reaction: 20 mmol scale No flash chromatography! 18 Surprise Slide! Happy birthday Mylène! 19 Conclusions •Monofluorination: Mild reaction conditions Broad scope •Difluorination: Mild reaction conditions First catalytic C-H difluorination •General Use of visible light Application to gram scale reactions No transition-metal required •Further improvements: Use of continuous-flow conditions Application to the synthesis of a drug 20











