Photoredox Organocatalysis
advertisement
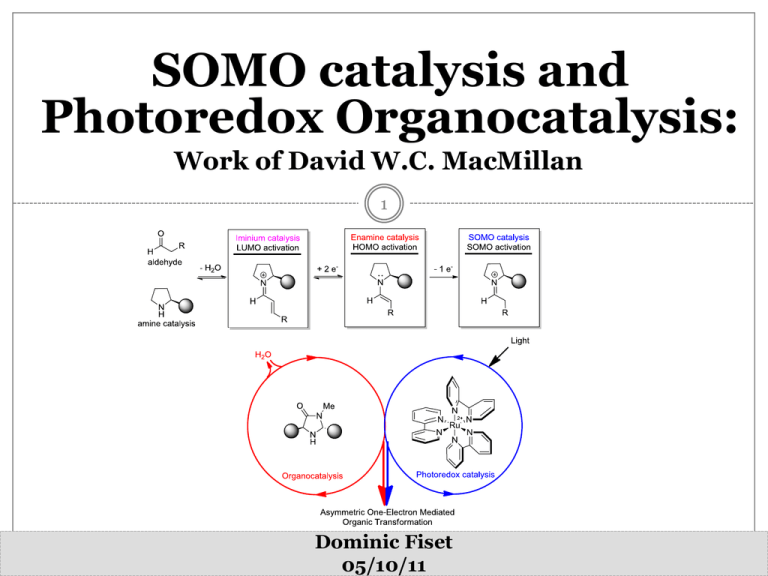
SOMO catalysis and Photoredox Organocatalysis: Work of David W.C. MacMillan 1 Dominic Fiset 05/10/11 About Me 2 RIP 1972-1995 About Me 3 Outline 4 Introduction David W.C. MacMillan First Developments in Organocatalysis Aminocatalysis: Activation modes Singly-Occupied Molecular Orbital catalysis Previously Reported Radical Alkylation Mechanistic Considerations Scope and Limitations Photoredox Organocatalysis Merging SOMO Catalysis with Photoredox Chemistry Mechanistic Considerations Synthetic Utility Pr. David W.C. MacMillan 5 Biography - Born in Bellshill, Scotland, in 1968 - 1987-91: Undergrad with Dr. Ernie Colvin at the University of Glasgow - 1991- 96: PhD. with Professor Larry E. Overman - 1996-98: Postdoctoral research fellow with Professor David E. Evans - 1998-2000: Independent Research at University of California, Berkeley -2000-2006: Professor of Chemistry at the California Institute of Technology -2006-...: Professor of Chemistry at Princeton University -2o10-…: Editor-in-Chief of Chemical Science published by RSC Research Interests - Organocatalysis - Mechanist investigation - SOMO catalysis - Merging photoredox catalysis and organocatalysis - Total synthesis of complex natural products 1. h http://www.princeton.edu/chemistry/macmillan/index.xml Birth of Organocatalysis 6 Hajos-Parrisch-Eder-Sauer-Wiechert reaction (1970s) 1. (a) Cheong, P. H.-Y.; Legault, C. Y.; Um, J. M.; Çelebi-Ö lçü m, N.; Houk, K. N. Chem. Rev. 2011, 111, 5042 (b) Hajos, A. G.; Parrish, D. R. J. Org. Chem. 1974, 39, 1612 (c) Eder, U.; Sauer, G.; Wiechert, R. Angew. Chem., Int. Ed. Engl. 1971, 10, 496. Advent of Mordern Organocatalysis: Asymmetric Aminocatalysis 7 Enamine catalysis: Aldol reaction Iminium catalysis: Diels-Alder reaction 1. (a) List, B.; Lerner, R. A.; Barbas, C. F., III J. Am. Chem. Soc. 2000, 122, 2395. (b) List, B. Synlett 2001, 1675 2. Ahrendt, K. A.; Borths, C. J.; MacMillan, D. W. C. J. Am. Chem. Soc. 2000, 122, 4243 Asymmetric aminocatalysis: Activation Modes 8 1. (a) Grondal, C.; Jeanty, M.; Enders, D. Nat .Chem. 2010, 2, 167. (b) MacMillan, D. W. C. Nature 2008, 455, 304 The Holy Grail Reaction 9 Pioneering work by List and Córdeva Catalytic asymmetric intermolecular α-alkylation of aldehydes 1. 2. (a) Vignola, N.; List, B. J. Am. Chem. Soc. 2003, 126, 450 (b) Ibrahem, I.; Córdova, A. Angew. Chem. Int. Ed. 2006, 45, 1952 Melchiorre, P. Angew. Chem. Int. Ed. 2009, 48, 1360 The Holy Grail Reaction: A New Activation Mode 10 Asymmetric intermolecular α-alkylation of aldehyde Is there a solution ? 1. MacMillan, D.W.C. Lecture 4: New acctivation mode (SET pathways), available online at http://www.princeton.edu/chemistry/macmillan/research/MacMillan%20Lecture%204.pdf A New Activation Mode: Singly-Occupied Molecular Orbital Catalysis 11 Aminocatalysis: A new activation mode 1 SOMO catalysis: A new synthetic paradigm 1. Beeson, T. D.; Mastracchio, A.; Hong, J.-B.; Ashton, K.; MacMillan, D. W. C. Science 2007, 316, 582 Enamine oxidation: Racemic SOMO catalysis 12 Stereoselective addition of radicals to chiral enamines: work of Shubert Cation radicals of enamines: work of Murakami and coworkers Construction of quaternary center: work of Cossy 1. 2. 3. Renaud, P.; Schubert, S. Synlett 1990, 624 Narasaka, K.; Okauchi, T.; Tanaka, K.; Murakami, M. Chem. Lett. 1992, 21, 2099 (a) Cossy, J.; Bouzide, A. J. Chem. Soc., Chem. Commun. 1993, 1218 (b) Cossy, J.; Bouzide, A.; Leblanc, C. Synlett 1993, 202 SOMO catalysis: Work of D.W.C. MacMillan 13 1. Beeson, T. D.; Mastracchio, A.; Hong, J.-B.; Ashton, K.; MacMillan, D. W. C. Science 2007, 316, 582 Intermolecular Allylation of Aldehydes 14 1. Beeson, T. D.; Mastracchio, A.; Hong, J.-B.; Ashton, K.; MacMillan, D. W. C. Science 2007, 316, 582 Potential for a Broad Scope 15 1. Beeson, T. D.; Mastracchio, A.; Hong, J.-B.; Ashton, K.; MacMillan, D. W. C. Science 2007, 316, 582 SOMO Catalysis: Evidence for Radical Pathway 16 1. (a) Beeson, T. D.; Mastracchio, A.; Hong, J.-B.; Ashton, K.; MacMillan, D. W. C. Science 2007, 316, 582 (b) Le Tadic-Biadatti, M.-H.; Newcomb, M. Journal of the Chemical Society, Perkin Transactions 2 1996, 1467 Organo-SOMO Catalysis 17 1. (a) Beeson, T. D.; Mastracchio, A.; Hong, J.-B.; Ashton, K.; MacMillan, D. W. C. Science 2007, 316, 582 (b) Le Tadic-Biadatti, M.-H.; Newcomb, M. Journal of the Chemical Society, Perkin Transactions 2 1996, 1467 Chemoselective Oxidation 18 1. (a) Devery, J. J.; Conrad, J. C.; MacMillan, D. W. C.; Flowers, R. A. Angew. Chem. Int. Ed. 2010, 49, 6106 (b) Beel, R.; Kobialka, S.; Schmidt, M. L.; Engeser, M. Chem. Commun. 2011, 47, 3293 (c) Um, J. M.; Gutierrez, O.; Schoenebeck, F.; Houk, K. N.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 6001 Origin of the Enantioselectivity 19 Steric Control Approach 1. Beeson, T. D.; Mastracchio, A.; Hong, J.-B.; Ashton, K.; MacMillan, D. W. C. Science 2007, 316, 582 Catalytic Turnovers 20 Water Plays a Key Role - Concentration of catalyst is maintained by H2O (below 2.00 eq, the catalyst is deactivated) - Effect on the phase-transfer process that controls the homogenous concentration of the oxidant (CAN) - Bench-grade DME contains sufficient water to achieve optimal results 1. (a) Beeson, T. D.; Mastracchio, A.; Hong, J.-B.; Ashton, K.; MacMillan, D. W. C. Science 2007, 316, 582 (b) Devery, J. J.; Conrad, J. C.; MacMillan, D. W. C.; Flowers, R. A. Angew. Chem. Int. Ed. 2010, 49, 6106 SOMO catalysis: Work of M. P. Sibi 21 1. (a) Sibi, M. P.; Hasegawa, M. J. Am. Chem. Soc. 2007, 129, 4124 (b) Van Humbeck, J. F.; Simonovich, S. P.; Knowles, R. R.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 10012 Work of M. P. Sibi: Revisited by MacMillan 22 1. (a) Sibi, M. P.; Hasegawa, M. J. Am. Chem. Soc. 2007, 129, 4124 (b) Van Humbeck, J. F.; Simonovich, S. P.; Knowles, R. R.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 10012 Work of M. P. Sibi: Revisited by MacMillan 23 Conditions Recently Re-Optimized by MacMillan: ‘’Synergistic catalysis’’ 1. (a) Van Humbeck, J. F.; Simonovich, S. P.; Knowles, R. R.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 10012 (b) Simonovich, S. P.; Van Humbeck, J. F.; MacMillan, D. W. C. Chemical Science 2011, ASAP, DOI: 10.1039/C1SC00556A a-Enolation of Aldehydes 24 1. Jang, H. Y.; Hong, J. B.; MacMillan, D. W. C. J. Am. Chem. Soc. 2007, 129, 7004 a-Vinylation of Aldehydes: Mechanism 25 1. Kim, H.; MacMillan, D. W. C. J. Am. Chem. Soc. 2008 130, 39 a-Vinylation of Aldehydes: Scope 26 Synthetic Application 1. Kim, H.; MacMillan, D. W. C. J. Am. Chem. Soc. 2008 130, 39 Carbo-oxidation of Styrenes 27 1. Graham, T. H.; Jones, C. M.; Jui, N. T.; MacMillan, D. W. C. J. Am. Chem. Soc. 2008, 130, 16494. Carbo-oxidation of Styrenes 28 Rapid Acess to Heterocyclic Rings Homobenzylation of aldehyde 1. Graham, T. H.; Jones, C. M.; Jui, N. T.; MacMillan, D. W. C. J. Am. Chem. Soc. 2008, 130, 16494. Organo-SOMO Cascade Cycloadditions 29 1. Jui, N. T.; Lee, E. C. Y.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 10015 Organo-SOMO Cascade Cycloadditions 30 1. Jui, N. T.; Lee, E. C. Y.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 10015 Organo-SOMO Cascade Cycloadditions 31 1. Jui, N. T.; Lee, E. C. Y.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 10015 Intramolecular a-Allylation 32 Catalyst –controlled stereoselective piperidine formation 1. Pham, P. V.; Ashton, K.; MacMillan, D. W. C. Chemical Science 2011, 2, 1470 Intramolecular a-Arylation of Aldehydes 33 1. (a) Nicolaou, K. C.; Reingruber, R. d.; Sarlah, D.; Brä se, S. J. Am. Chem. Soc. 2009, 131, 6640 (b) Conrad, J. C.; Kong, J.; Laforteza, B. N.; MacMillan, D. W. C. J. Am. Chem. Soc. 2009, 131, 11640 (c) Um, J. M.; Gutierrez, O.; Schoenebeck, F.; Houk, K. N.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 6001 a-Chlorination and Terminal Epoxide Formation 34 1. Amatore, M.; Beeson, T. D.; Brown, S. P.; MacMillan, D. W. C. Angew. Chem. Int. Ed. 2009, 48, 5121 a-Chlorination and Terminal Epoxide Formation 35 1. Amatore, M.; Beeson, T. D.; Brown, S. P.; MacMillan, D. W. C. Angew. Chem. Int. Ed. 2009, 48, 5121 a-Chlorination and Terminal Epoxide Formation 36 1. Amatore, M.; Beeson, T. D.; Brown, S. P.; MacMillan, D. W. C. Angew. Chem. Int. Ed. 2009, 48, 5121 a-Allylation of Ketones 37 1. (a) Northrup, A. B.; MacMillan, D. W. C. J. Am. Chem. Soc. 2002, 124, 2458 (b) Mastracchio, A.; Warkentin, A. A.; Walji, A. M.; MacMillan, D. W. C. Proceedings of the National Academy of Sciences 2010, 107, 20648 Polyene Cyclization 38 1. (a) MacMillan, D.W.C. Lecture 4: New acctivation mode (SET pathways), available online at http://www.princeton.edu/chemistry/macmillan/research/MacMillan%20Lecture%204.pdf (b) Rendler, S.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 5027 Polyene Cyclization: Mechanism 39 1. (a) MacMillan, D.W.C. Lecture 4: New acctivation mode (SET pathways), available online at http://www.princeton.edu/chemistry/macmillan/research/MacMillan%20Lecture%204.pdf (b) Rendler, S.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 5027 Polyene Cyclization: Scope 40 1. Rendler, S.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 5027 Merging SOMO and Photoredox catalysis 41 Reversing the role of the aminocatalyst 1. Rendler, S.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 5027 Light Photoredox Catalysis 42 1 Narayanam, J. M. R.; Stephenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102 Light Photoredox Catalysis 43 1 Juris, A.; Balzani, V.; Barigelletti, F.; Campagna, S.; Belser, P.; von Zelewsky, A. Coord. Chem. Rev. 1988, 84, 85 Merging SOMO and Photoredox catalysis 44 1 Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77 Merging SOMO and Photoredox catalysis 45 1 Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77 Photoredox organocatalysis: Mechanism 46 1 Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77 Photoredox Organocatalysis: Control Experiments 47 - Rigorous exclusion of light: No alkylation product - Removal of Ru(bpy)32+: <10% of alkylation product over an extended timeframe (24h) - Ru(bpy)32+ can be replaced by high-energy UV irradiation source Reaction efficiency over 80% - Fluorescent quenching experiments with Ru(bpy)32+* Ru(bpy)32+* excited state behaves as an oxidant in the photoredox cycle 1 Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77 Enantioselective a-Trifluoromethylation 48 1 Nagib, D. A.; Scott, M. E.; MacMillan, D. W. C. J. Am. Chem. Soc. 2009, 131, 10875 Enantioselective a-Trifluoromethylation 49 1 Nagib, D. A.; Scott, M. E.; MacMillan, D. W. C. J. Am. Chem. Soc. 2009, 131, 10875 Enantioselective a-Benzylation 50 1 Shih, H.-W.; Vander Wal, M. N.; Grange, R. L.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 13600 Enantioselective a-Benzylation: Mechanism 51 1 Shih, H.-W.; Vander Wal, M. N.; Grange, R. L.; MacMillan, D. W. C. J. Am. Chem. Soc. 2010, 132, 13600 Conclusion 52 1. MacMillan, D.W.C. Lecture 4: New acctivation mode (SET pathways), available online at http://www.princeton.edu/chemistry/macmillan/research/MacMillan%20Lecture%204.pdf