File
advertisement

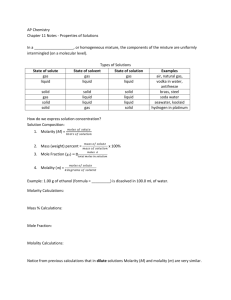
Exam 4 on Chapters 10 & 11 Chapter 13 notes Section 13.1 Section 13.2 Section 13.3 Section 13.4 Section 13.5 Section 13.6 Recall: A solution is a homogeneous mixture Made up of a solvent & one or more solutes The amount of solute that can be dissolved in a given solvent at a specific temperature Unsaturated: less than the max amount Saturated: the max amount Supersaturated: more than the max amount The process of solvent molecules breaking apart solute particles Solvation Process Solvation depends on 3 types of interactions Solute-Solute interactions Solvent-Solvent interactions Solute-Solvent interactions Recall: In Chapter 12 we talked about intermolecular forces that existed between molecules, atoms, or ions of a pure substance London Dispersion Dipole-Dipole Ion-Ion forces Because mixtures have different properties w/in, there are more forces that can be present When solute and solvent mix to make solutions, it has an associated enthalpy change known as ΔHsoln. ΔHsoln = ΔH1 + ΔH2 + ΔH3 When ΔHsoln > 0 When ΔHsoln < 0 Endothermic Exothermic Which of the following compounds do you expect to be more soluble in benzene than in water? SO2, CO2, Na2SO4, C2H6, Br2 Recall: concentration shows how much solute is in a solution or solvent Molarity: (M) moles of solute/L of solution Mole Fraction: (X) moles of solute/moles of solution Two additional concentrations: Molality (m) Percent by Mass Molality Moles of solute/kg of solvent Percent by Mass (Mass of solute/mass of solution) x 100 ▪ Percent means “parts per hundred” ▪ If we multiplied by 1000, it would be “parts per thousand” ▪ 1,000,000 (parts per million, ppm) ▪ 1,000,000,000 (parts per billion, ppb) Determine the percent by mass of KCl in a solution prepared by dissolving 1.18 g of KCl in 86.3 g of water. What is the molality of a solution prepared by dissolving 6.44 g of naphthalene (C10H8) in 80.1 g of benzene? Two factors affect the solubility of solutes in solutions: Temperature Pressure As temperature rises, most solids solubility increases As temperature rises, gases solubility decreases Pressure doesn’t affect solids or liquids much Gases are affected much more by pressure Henry’s Law ▪ The solubility of a gas in a liquid is proportional to the pressure of the gas Properties that depend ONLY on the number of solute particles in solution Vapor-Pressure Lowering Boiling-Point Elevation Freezing-Point Depression Osmotic Pressure Vapor pressure is the pressure of the vapor above a liquid (or solid) at equilibrium When a solute is added to a solvent, the vapor pressure of the solvent (above the resulting liquid) is LOWER than that of the pure solvent. Raoult’s Law Boiling Point: temperature at which a pure substance will vaporize (or boil) Where vapor pressure = atmospheric pressure Because adding solute lowers vapor pressure, more temperature is necessary to get vapor pressure to equal atmospheric pressure Boiling Point Elevates Freezing Point: the temperature at which a substance freezes The presence of a solute with a solvent lowers (or depresses) the temperature More energy has to be removed in a solution than in a pure substance Osmosis is the movement of solvent particles (through a semipermeable membrane) from a more dilute solution to a more concentrated solution Osmotic pressure: Pressure required by a solution to stop osmosis To calculate how the boiling point is elevated: ΔTb = Kbm where ΔTb is the boiling point elevation Kb is molal boiling-point elevation constant m is the molality of the solution To calculate how the freezing point is depressed: ΔTf = Kfm where ΔTf is the freezing point depression Kf is molal freezing-point depression constant m is the molality of the solution Determine the boiling point and the freezing point of a solution prepared by dissolving 678 g of glucose in 2.0 kg of water. For water, Kb = 0.52°C/m and Kf = 1.86 °C/m Calculate the freezing point and boiling point of a solution containing 268 g of ethylene glycol (C2H6O2) in 1015 g of water.