Document
advertisement

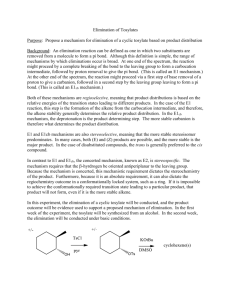
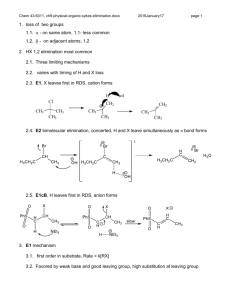
Elimination reactions Chapter 6 1 Elimination reactions A problem that often occurs in substitution reactions is elimination 2 E1-elimination n = k [substrate] The mechanism is similar to that of the SN1 reaction: the first step is formation of the carbocation via heterolytic cleavage 3 Substitution vs elimination Elimination and substitution reactions are competing processes: in this case, the tertiary carbocation gives • primarily elimination in the presence of a strong base (EtO–) • mainly substitution if there is only a good nucleophile present This can be considered as a ‘rule of thumb’ 4 pKa’s revisited Acid HI H3O + HF CH3CO2H H2 S NH4+ MeOH HCCH H2C=CH2 pKa –10 –1.7 3.2 4.2 7.0 9.4 15.2 25 45 Base CH3CO2¯ CN ¯ NH3 (CH3CH2)3N CH3O¯ HO¯ CH3CH2O¯ (CH3)3CO¯ NH2¯ pK* 4.2 9.1 9.4 10.7 15.2 15.7 15.9 19 35 *The pK values are those of the conjugated acids If the pK of the conjugated base is higher than the pKa of the acid deprotonation is possible (pK > 12-15: strong base) 5 The Saytzeff elimination Saytzeff elimination: in the elimination process, formation of the most substituted double bond is favored 6 Explanation In the transition state for elimination, the increased stability of the most substituted double bond is already felt so that there is a lower energy barrier for elimination of Ha 7 Problems 6.20: Predict the products of the E1 and SN1 reactions of the following molecules in water: 8 The E2 elimination n = k [substrate] [base] As in the SN2 reaction, the rate is dependent on the concentration of both reaction partners 9 E2 vs SN2 There is a strong similarity between the E2 and SN2 reactions: strong nucleophiles favor substitution, while strong bases favor elimination 10 Steric bulk favors elimination We already saw that SN2 substitution on a tertiary carbon is not possible, therefore E2 elimination will prevail (beside E1 elimination) 11 Other examples Note that at a primary carbon atom, only SN2 and no SN1 substitution is possible 12 Stereochemistry of the E2 rxn Possible orientations of the proton that eliminates and the leaving group 13 E2 and the anti-conformation The E2 elimination takes place in a single process in the anticonformation: proton abstraction, double bond formation and cleavage of the C–L bond occur simultaneously 14 Resemblance to SN2 The mechanism is ‘more or less’ analogous to the SN2 substitution The process is also called anti-elimination or antiperiplanar elimination 15 How to visualize this aspect ? What are the products of E2 elimination of both diastereomers ? 16 The answer….. 17 Regiochemistry Generally, formation of the most substituted double bond is favored Note the occurrence of E- and Z-isomers 18 Saytzeff vs Hofmann elimination 19 Influence of the base 20 Effect of the leaving group Especially quarternary ammonium leaving groups favor the Hofmann product 21 The E1cB elimination The E1cB reaction resembles the SN2 reaction, with the difference that there is an anion formed prior to the loss of the leaving group 22 The three eliminations E1cB: the proton is removed first, an anion is formed E1: the leaving group departs first, a cation is formed E2: all processes occur at the same time 23 Example of an E1cB reaction This reaction is possible if there is a group present that can stabilize the negative charge 24 Stability of anions The more substituted the carbanion, the less stable it is; this is a result of the inductively electron donating alkyl groups 25 The synthetic outlook 26 Formation of CC-bonds Formation of CC-bonds is a synthetically important reaction 27 Summary 28 Summary (2) 29 Problems Make problems: 6.30, 6.31, 6.32, 6.38, 6.53, 6.54, 6.56, 6.59 30