Lecture 2 of 5 Electrodeposition of Coating
advertisement

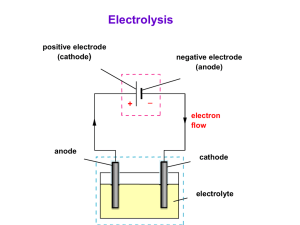
Outline Curriculum (5 lectures) Each lecture 45 minutes • Lecture 1: An introduction in electrochemical coating • Lecture 2: Electrodeposition of coating • Lecture 3: Anodizing of valve metal • Lecture 4: Electroless deposition of coating • Lecture 5: Revision in electrochemical coating Lecture 2 of 5 Electrodeposition of Coating Electrochemical Surface Engineering • • • • • An electro-chemical reaction Cathode: Metals/alloys coatings Anode: Soluble or insoluble Conductive solution: ionic species Transfer of electrons An example of electroplating of copper Power Supply Copper Anode e- Steel Cathode Main reaction Cu2+ + 2e- Cu Other possible electrochemical reactions At the cathode Electrodeposition of copper Cu2+ + 2e- Cu Hydrogen evolution 2H+ + 2e- H2 At the anode Soluble anode Dissolution of copper Insoluble anode Oxygen evolution Overall reaction Cu 2e- Cu2+ H2O 2e- 2H+ + 0.5 O2 Cu2+ + H2O Cu + 2H+ + 0.5 O2 Definition: Electron transfer reactions • Oxidizing agent + n e- = Reducing agent • Oxidizing agents get reduced • Reducing agents get oxidized • Oxidation is a loss of electrons (OIL) OILRIG • Reduction is a gain of electrons (RIG) Typical steps in the electroplating of metals 1. Cleaning with organic solvent or aqueous alkaline; to remove dirt or grease. 2. Is the surface is covered by oxides as a result of corrosion, clean with acid. 3. Rinse with water to neutralise the surface. 4. Electroplate metals under controlled condition. 5. Rinse with water and dry. 6. Additional step: heat treatment in air or vacuum environment What is the Job of the Bath? • Provides an electrolyte – to conduct electricity, ionically • Provides a source of the metal to be plated – as dissolved metal salts leading to metal ions • Allows the anode reaction to take place – usually metal dissolution or oxygen evolution • Wets the cathode work-piece – allowing good adhesion to take place • Helps to stabilise temperature – acts as a heating/cooling bath Typically, What is in a Bath? e.g., Watts Nickel • Ions of the metal to be plated, e.g. – Ni2+ (nickel ions) added mostly as the sulphate • Conductive electrolyte – NiSO4, boric acid, NiCl2 • Nickel anode dissolution promoter – NiCl2 provides chloride ions • pH buffer stops cathode getting too alkaline – Boric acid (H3BO3) • Additives – Wetters, levellers, brighteners, stress modifiers.. Current efficiency • pH changes accompany electrode reactions wherever H+ or OH- ions are involved. • In acid, hydrogen evolution occurs on the surface of cathode. This will result in a localised increase in pH near the surface of the electrode. 2H+ + 2e- H2 • In acid, oxygen evolution occurs on the surface of anode. This will result in a drop of pH near the surface of the electrode. H2O 2e- 2H+ + 0.5 O2 H+ • pH buffer stops the cathode getting too alkaline. – Boric acid (H3BO3) Cathode H2O H+ + OH H2 OH Current efficiency • Is the ratio between the actual amount of metal deposit, Ma to that calculated theoretically from Faradays Law, Mt. Current efficiency Ma Mt 100 % Parameters that may influence the quality of electrodeposits • • • • • • Current density (low to high current) The nature of anions/cations in the solution Bath composition, temperature, fluid flow Type of current waveform the presence of impurities physical and chemical nature of the substrate surface An example of Current vs. Potential Curve for electroplating of metal Typical Recipe and Conditions Watts Nickel Component Concentration/g L-1 Nickel sulphate Nickel chloride Boric acid Additives Temperature pH Current density 330 45 40 various 60 oC 4 2-10 A dm-2 Faraday’s Laws of Electrolysis Amount of material = amount of electrical energy n q [ mol ] zF n = amount of material q = electrical charge z = number of electrons F = Faraday constant [C ] [ C mol 1 ] Faraday’s Laws of Electrolysis: Expanded Relationship n q zF w M It zF n = amount of material w = mass of material M = molar mass of material I = current t = time z = number of electrons F = Faraday constant Current, Current density, Surface area j I A j = current density [mA cm-2] I = current [A] A = surface area of the electrode [cm2] jelectroplate = electroplating current density (metal electroplate) jcorrosion = corrosion current density (metal corrosion/dissolution) Faraday’s Laws of Electrolysis: Average thickness M .I .t w = weight (mass) of metal w M = molar mass of metal z .F I = current t = time M .I .t z = number of electrons x F = Faraday constant . A .z .F x = thickness of plating Faraday’s Laws of Electrolysis: Average deposit thickness x M .I .t . A . z .F The thickness of plate depends on: - the current (I) - the time for which it passes (t) - the exposed area of the work-piece (A) - a constant (M/AzF) which depends on the metal and the bath Faraday’s Laws of Electrolysis: Question - Nickel Plating Nickel is plated from a Watts bath at a current density of 3 A dm-2. The current efficiency is 96%. The molar mass of nickel is 58.71 g mol-1. The density of nickel is 8.90 g cm-3. The Faraday constant is 96 485 C mol-1. What will be the averaged plating thickness in 1 hour? Faraday’s Laws of Electrolysis: Answer - Nickel Plating Assume that the reaction is: Ni2+ + 2e- = Ni So, two electrons are involved for every Ni atom, and z = 2 The current density used in plating nickel is 96% of the total current, i.e., 0.96 x 3 A dm-2. Faraday’s Laws of Electrolysis: Answer - Nickel Plating The average deposit thickness is given by: x x M .I .t . A . z .F ( 58 . 71 g mol ( 8 . 90 g cm x 3 . 54 x 10 3 1 )( 0 . 96 x 3 A )( 3600 s ) 2 )( 100 cm )( 2 )( 96485 C mol 3 cm 35 . 4 x 10 4 1 ) cm 35 m Summary • Electrodeposition is a versatile coating technique. • There is a high degree of control over deposit thickness. • Many metals can be electroplated from aqueous baths. • So can some alloys, conductive polymers and composites. • Rates of electroplating can be expressed via Faraday’s Laws of electrolysis. Thank you for your attention!